Dermatologic conditions in women receiving systemic cancer therapy
- PMID: 31909148
- PMCID: PMC6938835
- DOI: 10.1016/j.ijwd.2019.10.003
Dermatologic conditions in women receiving systemic cancer therapy
Abstract
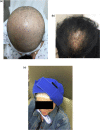
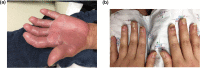
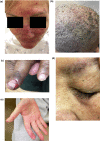
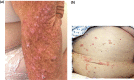
As advances in cancer therapies have improved cancer-related survival, novel therapeutics have also introduced a variety of dermatologic toxicities, and an increased number of patients are living with these sequalae. Women with cancer in particular experience a spectrum of dermatologic conditions that affect their skin, hair, nail, and mucosal surfaces. Studies have shown that these toxic effects can significantly affect quality of life and alter a woman's self-image, cultural identity, femininity, sexuality, and mental health. In severe instances, dermatologic toxicities may even disrupt cancer therapy and can therefore affect overall survival and treatment response. In this article, we review the dermatologic adverse effects from traditional chemotherapy, targeted therapy, immune checkpoint inhibitors, and endocrine therapy that disproportionately affect women. The timely diagnosis and management of these dermatologic conditions is crucial in the multidisciplinary care of women with cancer.
Keywords: Cancer therapy; Cutaneous adverse effects; Dermatologic toxicities; Oncodermatology; Women’s health.
© 2019 Published by Elsevier Inc. on behalf of Women's Dermatologic Society.
Figures

References
-
- Al-Batran S.E., Meerpohl H.G., von Minckwitz G., Atmaca A., Kleeberg U., Harbeck N. Reduced incidence of severe palmar-plantar erythrodysesthesia and mucositis in a prospective multicenter phase II trial with pegylated liposomal doxorubicin at 40 mg/m2 every 4 weeks in previously treated patients with metastatic breast cancer. Oncology. 2006;70(2):141–146. - PubMed
-
- Alexandrescu D.T., Kauffman C.L., Dasanu C.A. Persistent hair growth during treatment with the EGFR inhibitor erlotinib. Dermatol Online J. 2009;15(3):4. - PubMed
-
- Archer D.F., Sturdee D.W., Baber R., de Villiers T.J., Pines A., Freedman R.R. Menopausal hot flushes and night sweats: Where are we now? Climacteric. 2011;14(5):515–528. - PubMed
-
- Balagula Y., Rosen S.T., Lacouture M.E. The emergence of supportive oncodermatology: the study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol. 2011;65(3):624–635. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

