Effect of fluocinolone acetonide 0.2 μg/day implant on the decision to drive in patients with diabetic macular oedema: a report from the FAME study
- PMID: 31909197
- PMCID: PMC6936565
- DOI: 10.1136/bmjophth-2019-000405
Effect of fluocinolone acetonide 0.2 μg/day implant on the decision to drive in patients with diabetic macular oedema: a report from the FAME study
Abstract
Objective: This study aimed to determine whether treatment with the 0.2 µg/day fluocinolone acetone implant (FAc; ILUVIEN, Alimera Sciences) and the associated improvements in best-corrected visual acuity (BCVA) and central subfield thickness (CST) demonstrated in the Fluocinolone Acetonide in Diabetic Macular Edema (FAME) study have an impact on the patient's decision to drive as measured by the National Eye Institute Visual Functioning Questionnaire-25 (NEI-VFQ-25).
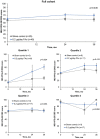
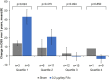
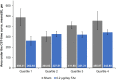
Methods: This was a post hoc analysis of up to 3 years of NEI-VFQ-25 data collected during the phase III FAME trial. Patients were divided into four quartiles according to baseline NEI-VFQ-25 driving subscale (DSS) score. Patients who had never driven were excluded. Patients received either the 0.2 µg/day FAc implant or sham (dummy injection). Change in the DSS score of the NEI-VFQ-25 questionnaire over 3 years in FAc-treated versus sham-treated patients was analysed by BCVA, CST and baseline DSS score.
Results: The proportion of patients achieving BCVA≥20/40 was similar between the FAc and sham groups throughout the study, while improvements in CST were significantly greater in the quartile of FAc-treated patients with the lowest baseline DSS score (quartile 1; p=0.04). Significant improvements in DSS score were also observed in quartile 1 (p=0.024), while numerical-but not significant-improvements in DSS score were observed in the full cohort.
Conclusion: This post hoc analysis demonstrates a significant association between clinical outcomes in diabetic macular oedema and improvement in quality of life measures following a single FAc implant.
Keywords: Retina.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DSG reports consultancy with Alimera Sciences and EyePoint Pharmaceuticals. SMH reports consultancy or speaker’s bureau membership with EyePoint Pharmaceuticals, Alimera Sciences, Ocular Therapeutix, Alcon, Allergan, OD-OS, Sandoz-Novartis, Spark Therapeutics, Regeneron and Clearside Biomedical. IJS reports consultancy or speaker’s bureau membership with Alimera Sciences, Allergan, Genentech, Novartis and Regeneron.
Figures

Similar articles
-
Effects of Long-Term DME Control With 0.2 µg/Day Fluocinolone Acetonide Implant on Quality of Life: An Exploratory Analysis From the FAME Trial.Ophthalmic Surg Lasers Imaging Retina. 2020 Nov 1;51(11):658-667. doi: 10.3928/23258160-20201104-10. Ophthalmic Surg Lasers Imaging Retina. 2020. PMID: 33231701
-
Comparison of data characterizing the clinical effectiveness of the fluocinolone intravitreal implant (ILUVIEN) in patients with diabetic macular edema from the real world, non-interventional ICE-UK study and the FAME randomized controlled trials.Curr Med Res Opin. 2019 Jul;35(7):1165-1176. doi: 10.1080/03007995.2018.1560779. Epub 2019 Jan 17. Curr Med Res Opin. 2019. PMID: 30569759
-
Fluocinolone Acetonide Intravitreal Implant 190 μg (ILUVIEN®) in Vitrectomized versus Nonvitrectomized Eyes for the Treatment of Chronic Diabetic Macular Edema.Ophthalmic Res. 2018;59(2):68-75. doi: 10.1159/000484091. Epub 2017 Dec 16. Ophthalmic Res. 2018. PMID: 29248913
-
Fluocinolone acetonide intravitreal implant (Iluvien®): in diabetic macular oedema.Drugs. 2013 Feb;73(2):187-93. doi: 10.1007/s40265-013-0010-x. Drugs. 2013. PMID: 23335133 Review.
-
Fluocinolone Acetonide Intravitreal Implant 0.19 mg (ILUVIEN®): A Review in Diabetic Macular Edema.Drugs. 2017 Apr;77(5):575-583. doi: 10.1007/s40265-017-0722-4. Drugs. 2017. PMID: 28283896 Review.
References
LinkOut - more resources
Full Text Sources
