The effectiveness of plant hydrocolloids at maintaining the quality characteristics of the encapsulated form of L-phenylalanine-ammonia-lyase
- PMID: 31909265
- PMCID: PMC6938834
- DOI: 10.1016/j.heliyon.2019.e03096
The effectiveness of plant hydrocolloids at maintaining the quality characteristics of the encapsulated form of L-phenylalanine-ammonia-lyase
Abstract
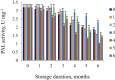
The effect of three types of polysaccharides (agar-agar, carrageenan, hydroxypropyl methylcellulose) on the activity and stability during storage at given temperature conditions of the enzyme preparation L-phenylalanine ammonia-lyase was studied. It was found that the most suitable storage temperature for encapsulated L-phenylalanine-ammonia-lyase is room temperature up to 25 °C for all samples of capsules from plant polysaccharides. Samples of capsules with agar-agar and hydroxypropyl methylcellulose under different temperature conditions inhibited the decrease in enzyme activity, which in other samples of capsules reached 90% in 6 months of storage. In samples of capsules with carrageenan at temperatures of 4 °C and 30 °C, there was a significant decrease in the activity of the enzyme preparation. Selection of capsule samples from plant polysaccharides suitable for L-phenylalanine-ammonia-lyase replacement therapy is done after studying the mechanisms of capsule destruction under conditions close to the conditions of the gastrointestinal tract, to which the next stage of our research will be devoted.
Keywords: Biotechnology; Capsules; Enzyme preparation; Natural polysaccharides; Pharmaceutical chemistry; Pharmaceutical science; Phenylalanine ammonia-lyase; Phenylketonuria; Stability.
© 2019 The Author(s).
Figures


Similar articles
-
Study of the Potential of the Capsule Shell Based on Natural Polysaccharides in Targeted Delivery of the L-Phenylalanine Ammonia-Lyase Enzyme Preparation.Pharmaceuticals (Basel). 2020 Apr 9;13(4):63. doi: 10.3390/ph13040063. Pharmaceuticals (Basel). 2020. PMID: 32283743 Free PMC article.
-
Study of the l-Phenylalanine Ammonia-Lyase Penetration Kinetics and the Efficacy of Phenylalanine Catabolism Correction Using In Vitro Model Systems.Pharmaceutics. 2021 Mar 13;13(3):383. doi: 10.3390/pharmaceutics13030383. Pharmaceutics. 2021. PMID: 33805682 Free PMC article.
-
Study on formulation and preparation technology of the composite cellulose-based enteric capsule shells.Drug Dev Ind Pharm. 2022 Nov;48(11):646-656. doi: 10.1080/03639045.2022.2152837. Epub 2022 Dec 16. Drug Dev Ind Pharm. 2022. PMID: 36469628
-
Phenylalanine ammonia lyase (PAL): From discovery to enzyme substitution therapy for phenylketonuria.Mol Genet Metab. 2018 Aug;124(4):223-229. doi: 10.1016/j.ymgme.2018.06.002. Epub 2018 Jun 9. Mol Genet Metab. 2018. PMID: 29941359 Review.
-
Phenylalanine ammonia lyase, enzyme substitution therapy for phenylketonuria, where are we now?Mol Genet Metab. 2005 Dec;86 Suppl 1:S22-6. doi: 10.1016/j.ymgme.2005.06.016. Epub 2005 Sep 13. Mol Genet Metab. 2005. PMID: 16165390 Review.
Cited by
-
Study of the Potential of the Capsule Shell Based on Natural Polysaccharides in Targeted Delivery of the L-Phenylalanine Ammonia-Lyase Enzyme Preparation.Pharmaceuticals (Basel). 2020 Apr 9;13(4):63. doi: 10.3390/ph13040063. Pharmaceuticals (Basel). 2020. PMID: 32283743 Free PMC article.
-
Study of the l-Phenylalanine Ammonia-Lyase Penetration Kinetics and the Efficacy of Phenylalanine Catabolism Correction Using In Vitro Model Systems.Pharmaceutics. 2021 Mar 13;13(3):383. doi: 10.3390/pharmaceutics13030383. Pharmaceutics. 2021. PMID: 33805682 Free PMC article.
References
-
- Wessel A., Rohr F. Inborn errors of metabolism. In: Sonneville K., Duggan C., editors. Manual of Pediatric Nutrition. fifth ed. People's Publishing House-USA; Shelton, Connecticut: 2014. pp. 469–472.
-
- Blau N., Van Spronsen F.J., Levy H.L. Phenylketonuria. Lancet. 2010;376:1417–1427. - PubMed
-
- Levy H.L., Sarkissian C.N., Scriver C.R. Phenylalanine ammonia lyase (PAL): from discovery to enzyme substitution therapy for phenylketonuria. Mol. Genet. Metab. 2018;124(4):223–229. - PubMed
-
- Longo N., Harding C.O., Burton B.K., Grange D.K., Vockley J., Wasserstein M., Rice G.M., Dorenbaum A., Neuenburg J.K., Musson D.G., Gu Z., Sile S. Single-dose, subcutaneous recombinant phenylalanine ammonia lyase conjugated with polyethylene glycol in adult patients with phenylketonuria: an open-label, multicentre, phase 1 dose-escalation trial. Lancet. 2014;384:37–44. - PMC - PubMed
-
- Sarkissian C.N., Gámez A., Wang L., Charbonneau M., Fitzpatrick P., Lemontt J.F., Zhao B., Vellard M., Bell S.M., Henschell C., Lambert A., Tsuruda L., Stevens R.C., Scriver C.R. Preclinical evaluation of multiple species of PEGylated recombinant phenylalanine ammonia lyase for the treatment of phenylketonuria. Proc. Natl. Acad. Sci. U.S.A. 2008;105:20894–20899. - PMC - PubMed
LinkOut - more resources
Full Text Sources

