CFTR Therapeutics Normalize Cerebral Perfusion Deficits in Mouse Models of Heart Failure and Subarachnoid Hemorrhage
- PMID: 31909302
- PMCID: PMC6939007
- DOI: 10.1016/j.jacbts.2019.07.004
CFTR Therapeutics Normalize Cerebral Perfusion Deficits in Mouse Models of Heart Failure and Subarachnoid Hemorrhage
Abstract
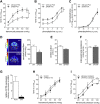
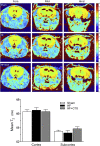
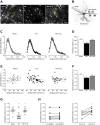
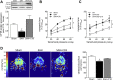
Heart failure (HF) and subarachnoid hemorrhage (SAH) chronically reduce cerebral perfusion, which negatively affects clinical outcome. This work demonstrates a strong relationship between cerebral artery cystic fibrosis transmembrane conductance regulator (CFTR) expression and altered cerebrovascular reactivity in HF and SAH. In HF and SAH, CFTR corrector compounds (C18 or lumacaftor) normalize pathological alterations in cerebral artery CFTR expression, vascular reactivity, and cerebral perfusion, without affecting systemic hemodynamic parameters. This normalization correlates with reduced neuronal injury. Therefore, CFTR therapeutics have emerged as valuable clinical tools to manage cerebrovascular dysfunction, impaired cerebral perfusion, and neuronal injury.
Keywords: CBF, cerebral blood flow; CFTR, cystic fibrosis transmembrane conductance regulator; HF, heart failure; MAP, mean arterial pressure; MOPS, 3-morpholinopropanesulfonic acid; MRI, magnetic resonance imaging; NIH, National Institutes of Health; PCA, posterior cerebral artery; S1P, sphingosine-1-phosphate; SAH, subarachnoid hemorrhage; TNF, tumor necrosis factor; TPR, total peripheral resistance; cognitive impairment; corrector compounds; cystic fibrosis transmembrane conductance regulator (CFTR); myogenic vasoconstriction; sphingosine-1-phosphate; tumor necrosis factor; vascular smooth muscle cells.
© 2019 The Authors.
Figures




Comment in
-
Targeting Muscles in the Brain to Enhance Cerebral Perfusion.JACC Basic Transl Sci. 2019 Dec 23;4(8):959-961. doi: 10.1016/j.jacbts.2019.11.003. eCollection 2019 Dec. JACC Basic Transl Sci. 2019. PMID: 31909771 Free PMC article.
References
-
- Román G.C. Brain hypoperfusion: a critical factor in vascular dementia. Neurol Res. 2004;26:454–458. - PubMed
-
- Doehner W., Ural D., Haeusler K.G. Heart and brain interaction in patients with heart failure: overview and proposal for a taxonomy. A position paper from the Study Group on Heart and Brain Interaction of the Heart Failure Association. Eur J Heart Fail. 2018;20:199–215. - PubMed
-
- Wong G.K., Lam S., Ngai K. Evaluation of cognitive impairment by the Montreal cognitive assessment in patients with aneurysmal subarachnoid haemorrhage: prevalence, risk factors and correlations with 3 month outcomes. J Neurol Neurosurg Psychiatry. 2012;83:1112–1117. - PubMed
-
- Yang J., Hossein Noyan-Ashraf M., Meissner A. Proximal cerebral arteries develop myogenic responsiveness in heart failure via tumor necrosis factor-α-dependent activation of sphingosine-1-phosphate signaling. Circulation. 2012;126:196–206. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

