Design, Synthesis, and Targeted Delivery of Fluorescent 1,2,4-Triazole-Peptide Conjugates to Pediatric Brain Tumor Cells
- PMID: 31909311
- PMCID: PMC6941177
- DOI: 10.1021/acsomega.9b01903
Design, Synthesis, and Targeted Delivery of Fluorescent 1,2,4-Triazole-Peptide Conjugates to Pediatric Brain Tumor Cells
Abstract
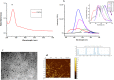
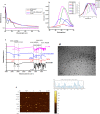
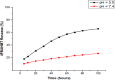
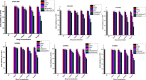
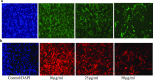
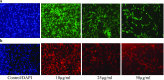
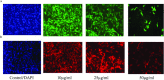
Most of the chemotherapeutics and drug-delivery models pose serious health problems and several undesirable side effects due to nonspecificity, lack of proper targeting system, and their large sizes. The rational design and synthesis of target-specific chemotherapeutics are highly important. This research work is focused on the rational design, synthesis, and anticancer studies of fluorescent 1,2,4-triazole-peptide conjugates for the development of target-specific anticancer drugs. Three novel 1,2,4-triazole derivatives: 4-(4-fluorobenzylidenamino)-3-hydrazino-5-mercapto-1,2,4-triazole (4FBAHMT, 2a), 4-(3,4,5-trimethoxybenzylidenamino)-3-hydrazino-5-mercapto-1,2,4-triazole (TMOBAHMT, 2b), and 4-(4-benzyloxy-2-methyloxbenzylidenamino)-3-hydrazino-5-mercapto-1,2,4-triazole (4BO2MOBAHMT, 2c) were synthesized after screening through molecular docking procedures. The docking studies were performed between ligand molecules and αvβ6 integrin protein. Fluorescent carbon nanoparticles (CNPs, 3) were conjugated with 1,2,4-triazole derivatives (2a-c) and l-carnosine (LC) dipeptide to get their corresponding conjugates (4a-c). The title double conjugates were characterized by spectroscopic (UV/vis spectroscopy, fluorescence spectroscopy, and FTIR spectroscopy) and microscopic (scanning electron microscopy, transmission electron microscopy, and atomic force microscopy) techniques. In vitro efficacy of fluorescent 1,2,4-triazole-peptide conjugates was investigated against two pediatric brain tumor cell lines (CHLA-200 & SJGBM2) and human embryonic kidney cell line (HEK293 as a control) by employing cell proliferation assay/MTS assay and fluorescence microscopy. 1,2,4-Triazole derivatives and their conjugates showed potent and selective anticancer activity against CHLA-200 and SJGBM2 cell lines. Cell proliferation assay and fluorescence microscopy results revealed that conjugates were more highly selective and cytotoxic than control drug temozolomide (TM) against both cell lines. CNPs are highly biocompatible and the quantum-sized conjugates were nontoxic for normal embryonic kidney cell line (HEK 293). The experimental results of MTS bioactivity assay and fluorescence microscopy were in close agreement with the theoretical results of molecular docking studies.
Copyright © 2019 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Ranganathan R.; Madanmohan S.; Kesavan A.; Baskar G.; Krishnamoorthy Y. R.; Santosham R.; Ponraju D.; Rayala S. K.; Venkatraman G. Nanomedicine: Towards development of patient-friendly drug-delivery systems for oncological applications. Int. J. Nanomed. 2012, 7, 1043–1060. 10.2147/IJN.S25182. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
