The genetic basis of aneuploidy tolerance in wild yeast
- PMID: 31909711
- PMCID: PMC6970514
- DOI: 10.7554/eLife.52063
The genetic basis of aneuploidy tolerance in wild yeast
Abstract
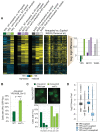
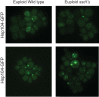
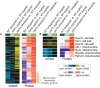
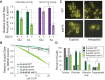
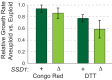
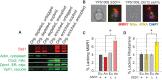
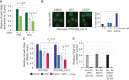
Aneuploidy is highly detrimental during development yet common in cancers and pathogenic fungi - what gives rise to differences in aneuploidy tolerance remains unclear. We previously showed that wild isolates of Saccharomyces cerevisiae tolerate chromosome amplification while laboratory strains used as a model for aneuploid syndromes do not. Here, we mapped the genetic basis to Ssd1, an RNA-binding translational regulator that is functional in wild aneuploids but defective in laboratory strain W303. Loss of SSD1 recapitulates myriad aneuploidy signatures previously taken as eukaryotic responses. We show that aneuploidy tolerance is enabled via a role for Ssd1 in mitochondrial physiology, including binding and regulating nuclear-encoded mitochondrial mRNAs, coupled with a role in mitigating proteostasis stress. Recapitulating ssd1Δ defects with combinatorial drug treatment selectively blocked proliferation of wild-type aneuploids compared to euploids. Our work adds to elegant studies in the sensitized laboratory strain to present a mechanistic understanding of eukaryotic aneuploidy tolerance.
Keywords: S. cerevisiae; aneuploidy; chromosomes; gene expression; natural variation; proteotoxicity; wild strains.
© 2020, Hose et al.
Conflict of interest statement
JH, LE, KC, HD, DR, VB, JC, ES, AG No competing interests declared
Figures


References
-
- Astuti D, Morris MR, Cooper WN, Staals RH, Wake NC, Fews GA, Gill H, Gentle D, Shuib S, Ricketts CJ, Cole T, van Essen AJ, van Lingen RA, Neri G, Opitz JM, Rump P, Stolte-Dijkstra I, Müller F, Pruijn GJ, Latif F, Maher ER. Germline mutations in DIS3L2 cause the Perlman syndrome of overgrowth and Wilms tumor susceptibility. Nature Genetics. 2012;44:277–284. doi: 10.1038/ng.1071. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

