Provision of Lipid-Based Nutrient Supplements to Mothers During Pregnancy and 6 Months Postpartum and to Their Infants from 6 to 18 Months Promotes Infant Gut Microbiota Diversity at 18 Months of Age but Not Microbiota Maturation in a Rural Malawian Setting: Secondary Outcomes of a Randomized Trial
- PMID: 31909811
- PMCID: PMC7138685
- DOI: 10.1093/jn/nxz298
Provision of Lipid-Based Nutrient Supplements to Mothers During Pregnancy and 6 Months Postpartum and to Their Infants from 6 to 18 Months Promotes Infant Gut Microbiota Diversity at 18 Months of Age but Not Microbiota Maturation in a Rural Malawian Setting: Secondary Outcomes of a Randomized Trial
Abstract
Background: Diet may alter the configuration of gut microbiota, but the impact of prenatal and postnatal nutritional interventions on infant gut microbiota has not been investigated.
Objective: We evaluated whether providing lipid-based nutrient supplements (LNSs) to mother-infant dyads promotes a more diverse and mature infant gut microbiota, compared to maternal supplementation with multiple micronutrients (MMN) or iron and folic acid (IFA).
Methods: We enrolled 869 pregnant women in a randomized trial in Malawi. There were 3 study groups, with women receiving 1 MMN capsule daily during pregnancy and 6 mo postpartum, or 1 LNS sachet (20 g) daily during pregnancy and 6 mo postpartum, or 1 IFA capsule daily (during pregnancy) then a placebo daily (postpartum). Infants in the LNS group received LNS from 6 to 18 mo; infants in the other groups did not receive supplements. The infants' fecal microbiota were characterized by PCR amplification and sequencing of the bacterial 16S rRNA gene (variable region 4). The primary outcomes were microbiota α diversity and maturation [as microbiota-for-age z score (MAZ)]. Specific associations of taxa with intervention were established with indicator species analysis (ISA).
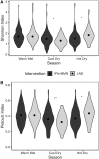
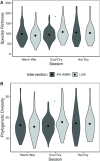
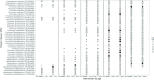
Results: Primary outcomes did not differ between IFA and MMN groups, so these groups were combined (IFA + MMN). Mean ± SD α diversity was higher in the LNS group at 18 mo for Shannon index [3.01 ± 0.57 (LNS) compared with 2.91 ± 0.60 (IFA + MMN), P = 0.032] and Pielou's evenness index [0.61 ± 0.08 (LNS) compared with 0.60 ± 0.09 (IFA + MMN), P = 0.043]; no significant differences were observed at 1, 6, 12, or 30 mo. MAZ and β diversity did not differ at any age. We found 10 and 3 operational taxonomic units (OTUs) positively associated with LNS and IFA + MMN, respectively; however, these associations became nonsignificant following false discovery rate correction at 10%.
Conclusions: Prenatal and postnatal LNS intake promoted infant gut microbiota diversity at 18 mo, after 12 mo of child supplementation, but did not alter microbiota maturation. This trial was registered at clinicaltrials.gov as NCT01239693.
Keywords: gut microbiota; infants diet; lipid-based nutrient supplements; microbiota diversity; multiple micronutrients.
Copyright © The Author(s) 2020.
Figures

Similar articles
-
Supplementation of Maternal Diets during Pregnancy and for 6 Months Postpartum and Infant Diets Thereafter with Small-Quantity Lipid-Based Nutrient Supplements Does Not Promote Child Growth by 18 Months of Age in Rural Malawi: A Randomized Controlled Trial.J Nutr. 2015 Jun;145(6):1345-53. doi: 10.3945/jn.114.207225. Epub 2015 Apr 29. J Nutr. 2015. PMID: 25926413 Clinical Trial.
-
Effects of maternal and child lipid-based nutrient supplements on infant development: a randomized trial in Malawi.Am J Clin Nutr. 2016 Mar;103(3):784-93. doi: 10.3945/ajcn.115.114579. Epub 2016 Feb 3. Am J Clin Nutr. 2016. PMID: 26843155 Clinical Trial.
-
Small-Quantity Lipid-Based Nutrient Supplements Increase Infants' Plasma Essential Fatty Acid Levels in Ghana and Malawi: A Secondary Outcome Analysis of the iLiNS-DYAD Randomized Trials.J Nutr. 2022 Jan 11;152(1):286-301. doi: 10.1093/jn/nxab329. J Nutr. 2022. PMID: 34543432
-
Vitamin and Mineral Supplementation During Pregnancy on Maternal, Birth, Child Health and Development Outcomes in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis.Nutrients. 2020 Feb 14;12(2):491. doi: 10.3390/nu12020491. Nutrients. 2020. PMID: 32075071 Free PMC article.
-
Effects of prenatal small-quantity lipid-based nutrient supplements on pregnancy, birth, and infant outcomes: a systematic review and meta-analysis of individual participant data from randomized controlled trials in low- and middle-income countries.Am J Clin Nutr. 2024 Oct;120(4):814-835. doi: 10.1016/j.ajcnut.2024.08.008. Epub 2024 Aug 16. Am J Clin Nutr. 2024. PMID: 39154665 Free PMC article.
Cited by
-
Iron Supplementation at the Crossroads of Nutrition and Gut Microbiota: The State of the Art.Nutrients. 2022 May 4;14(9):1926. doi: 10.3390/nu14091926. Nutrients. 2022. PMID: 35565894 Free PMC article. Review.
-
Community-Based Child Food Interventions/Supplements for the Prevention of Wasting in Children Up to 5 Years at Risk of Wasting and Nutritional Oedema: A Systematic Review and Meta-Analysis.Nutr Rev. 2025 Aug 1;83(8):1402-1424. doi: 10.1093/nutrit/nuaf041. Nutr Rev. 2025. PMID: 40272950 Free PMC article.
-
Colonization during a key developmental window reveals microbiota-dependent shifts in growth and immunity during undernutrition.Microbiome. 2024 Apr 9;12(1):71. doi: 10.1186/s40168-024-01783-3. Microbiome. 2024. PMID: 38589975 Free PMC article.
-
Associations between Gut Microbiota and Intestinal Inflammation, Permeability and Damage in Young Malawian Children.J Trop Pediatr. 2022 Feb 3;68(2):fmac012. doi: 10.1093/tropej/fmac012. J Trop Pediatr. 2022. PMID: 35149871 Free PMC article.
-
A Scoping Review Evaluating the Current State of Gut Microbiota Research in Africa.Microorganisms. 2023 Aug 20;11(8):2118. doi: 10.3390/microorganisms11082118. Microorganisms. 2023. PMID: 37630678 Free PMC article.
References
-
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R et al. .. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–51. - PubMed
-
- Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, Webb P, Lartey A, Black RE; Lancet Nutrition Interventions Review Group et al. .. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost?. Lancet. 2013;382:452–77. - PubMed
-
- Arimond M, Zeilani M, Jungjohann S, Brown KH, Ashorn P, Allen LH, Dewey KG. Considerations in developing lipid-based nutrient supplements for prevention of undernutrition: experience from the International Lipid-Based Nutrient Supplements (iLiNS) Project. Matern Child Nutr. 2015;11:(Suppl 4):31–61. - PMC - PubMed

