Factors associated with biomedical research participation within community-based samples across 3 National Cancer Institute-designated cancer centers
- PMID: 31909824
- PMCID: PMC7021578
- DOI: 10.1002/cncr.32487
Factors associated with biomedical research participation within community-based samples across 3 National Cancer Institute-designated cancer centers
Abstract
Background: Engaging diverse populations in biomedical research, including biospecimen donation, remains a national challenge. This study examined factors associated with an invitation to participate in biomedical research, intent to participate in biomedical research in the future, and participation in biomedical research and biospecimen donation among a diverse, multilingual, community-based sample across 3 distinct geographic areas.
Methods: Three National Cancer Institute-designated cancer centers engaged in community partnerships to develop and implement population health assessments, reaching a convenience sample of 4343 participants spanning their respective catchment areas. Data harmonization, multiple imputation, and multivariable logistic modeling were used.
Results: African Americans, Hispanic/Latinos, and other racial minority groups were more likely to be offered opportunities to participate in biomedical research compared to whites. Access to care, history of cancer, educational level, survey language, nativity, and rural residence also influenced opportunity, intent, and actual participation in biomedical research.
Conclusions: Traditionally underserved racial and ethnic groups reported heightened opportunity and interest in participating in biomedical research. Well-established community partnerships and long-standing community engagement around biomedical research led to a diverse sample being reached at each site and may in part explain the current study findings. However, this study illustrates an ongoing need to establish trust and diversify biomedical research participation through innovative and tailored approaches. National Cancer Institute-designated cancer centers have the potential to increase opportunities for diverse participation in biomedical research through community partnerships and engagement. Additional work remains to identify and address system-level and individual-level barriers to participation in both clinical trials and biospecimen donation for research.
Keywords: biospecimen donation; clinical trials; community engagement; disparities; diversity in research participation; prevention.
© 2020 American Cancer Society.
Conflict of interest statement
Figures

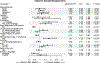
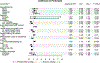
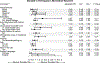

Comment in
-
An invitation for optimal inclusivity: Investing in communities to advance equity in biomedical research and cancer care.Cancer. 2020 Mar 1;126(5):935-938. doi: 10.1002/cncr.32683. Epub 2020 Jan 7. Cancer. 2020. PMID: 31909821 No abstract available.
References
-
- Al-Refaie WB, Vickers SM, Zhong W, Parsons H, Rothenberger D, Habermann EB. Cancer trials versus the real world in the United States. Annals of surgery. 2011;254(3):438–42; discussion 42–3. - PubMed
-
- Du W, Gadgeel SM, Simon MS. Predictors of enrollment in lung cancer clinical trials. Cancer. 2006;106(2):420–5. - PubMed
-
- Freedman LS, Simon R, Foulkes MA, Friedman L, Geller NL, Gordon DJ, et al. Inclusion of women and minorities in clinical trials and the NIH Revitalization Act of 1993--the perspective of NIH clinical trialists. Controlled clinical trials. 1995;16(5):277–85; discussion 86–9, 93–309. - PubMed
-
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. Jama. 2004;291(22):2720–6. - PubMed

