Regulatory Affairs 101: Introduction to Expedited Regulatory Pathways
- PMID: 31909876
- PMCID: PMC7214660
- DOI: 10.1111/cts.12745
Regulatory Affairs 101: Introduction to Expedited Regulatory Pathways
Abstract
Developing a novel drug, including discovery, nonclinical toxicology studies, initial clinical trials, and thorough pivotal studies, may take many years. Once an applicant has generated this comprehensive body of data, the final step prior to regulatory approval is Health Authority review of the marketing authorization application. Review by regulatory authorities to evaluate whether the data support a positive benefit/risk profile takes many months, adding additional time before patients may access therapy. In this paper, we discuss the various opportunities the US Food and Drug Administration and the European Medicines Agency offer to expedite the drug development and regulatory approval timelines for drugs intended to treat serious diseases and unmet medical needs.
© 2020 The Authors. Clinical and Translational Science published by Wiley Periodicals, Inc. on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures
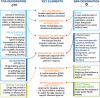
References
-
- US Food and Drug Administration (FDA) . Code of Federal Regulations Title 21 (21 CFR). Part 601, Subpart A–General Provisions and Subpart E–Accelerated approval of biological products for serious or life‐threatening illnesses and Part 314, Subpart B–Applications and Subpart H–Accelerated approval of new drugs for serious or life‐threatening illnesses. <https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm> (2018). Accessed June 25, 2019.
-
- US Food and Drug Administration (FDA) . Guidance for industry. Expedited programs for serious conditions – drugs and biologics. <https://www.fda.gov/files/drugs/published/Expedited-Programs-for-Serious...> (2014). Accessed June 25, 2019.
-
- European Medicines Agency (EMA) . Guideline on the scientific application and the practical arrangements necessary to implement the procedure for accelerated assessment pursuant to Article 14(9) of Regulation (EC) No 726/2004. <https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-sc...> (2016). Accessed June 25, 2019.
-
- US Food and Drug Administration (FDA) . Fast track. <https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated...> (2018). Accessed September 30, 2019.
-
- US Food and Drug Administration (FDA) . Frequently asked questions: breakthrough therapies. <https://www.fda.gov/regulatory-information/food-and-drug-administration-...> (2019). Accessed September 30, 2019.
MeSH terms
LinkOut - more resources
Full Text Sources

