Prevention of connexin-43 remodeling protects against Duchenne muscular dystrophy cardiomyopathy
- PMID: 31910160
- PMCID: PMC7108916
- DOI: 10.1172/JCI128190
Prevention of connexin-43 remodeling protects against Duchenne muscular dystrophy cardiomyopathy
Abstract
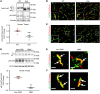
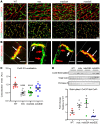
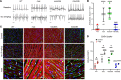
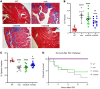
Aberrant expression of the cardiac gap junction protein connexin-43 (Cx43) has been suggested as playing a role in the development of cardiac disease in the mdx mouse model of Duchenne muscular dystrophy (DMD); however, a mechanistic understanding of this association is lacking. Here, we identified a reduction of phosphorylation of Cx43 serines S325/S328/S330 in human and mouse DMD hearts. We hypothesized that hypophosphorylation of Cx43 serine-triplet triggers pathological Cx43 redistribution to the lateral sides of cardiomyocytes (remodeling). Therefore, we generated knockin mdx mice in which the Cx43 serine-triplet was replaced with either phospho-mimicking glutamic acids (mdxS3E) or nonphosphorylatable alanines (mdxS3A). The mdxS3E, but not mdxS3A, mice were resistant to Cx43 remodeling, with a corresponding reduction of Cx43 hemichannel activity. MdxS3E cardiomyocytes displayed improved intracellular Ca2+ signaling and a reduction of NADPH oxidase 2 (NOX2)/ROS production. Furthermore, mdxS3E mice were protected against inducible arrhythmias, related lethality, and the development of cardiomyopathy. Inhibition of microtubule polymerization by colchicine reduced both NOX2/ROS and oxidized CaMKII, increased S325/S328/S330 phosphorylation, and prevented Cx43 remodeling in mdx hearts. Together, these results demonstrate a mechanism of dystrophic Cx43 remodeling and suggest that targeting Cx43 may be a therapeutic strategy for preventing heart dysfunction and arrhythmias in DMD patients.
Keywords: Cardiology; Cardiovascular disease; Cell Biology; Neuromuscular disease.
Conflict of interest statement
Figures


Comment in
-
A role for connexin-43 in Duchenne muscular dystrophy cardiomyopathy.J Clin Invest. 2020 Apr 1;130(4):1608-1610. doi: 10.1172/JCI135007. J Clin Invest. 2020. PMID: 32091412 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

