Use of Nanotrap particles for the capture and enrichment of Zika, chikungunya and dengue viruses in urine
- PMID: 31910225
- PMCID: PMC6946132
- DOI: 10.1371/journal.pone.0227058
Use of Nanotrap particles for the capture and enrichment of Zika, chikungunya and dengue viruses in urine
Abstract
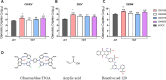
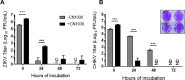
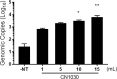
Nanotrap® (NT) particles are hydrogel microspheres developed for target analyte separation and discovery applications. NT particles consist of cross-linked N-isopropylacrylamide (NIPAm) copolymers that are functionalized with a variety of chemical affinity baits to enable broad-spectrum collection and retention of target proteins, nucleic acids, and pathogens. NT particles have been previously shown to capture and enrich arboviruses including Rift Valley fever and Venezuelan equine encephalitis viruses. Yet, there is still a need to enhance the detection ability for other re-emerging viruses such as Zika (ZIKV), chikungunya (CHIKV), and dengue (DENV) viruses. In this study, we exploited NT particles with different affinity baits, including cibacron blue, acrylic acid, and reactive red 120, to evaluate their capturing and enrichment capability for ZIKV, DENV and CHIKV in human fluids. Our results demonstrate that CN1030, a NT particle conjugated with reactive red 120, can recover between 8-16-fold greater genomic copies of ZIKV, CHIKV and DENV in virus spiked urine samples via RT-qPCR, superior to the other chemical baits. Also, we observed that CN1030 simultaneously enriched ZIKV, CHIKV and DENV in co-infection-based settings and could stabilize ZIKV, but not CHIKV infectivity in saliva spiked samples. CN1030 enriched viral detection at various viral concentrations, with significant enhancement observed at viral titers as low as 100 PFU/mL for ZIKV and 10 PFU/mL for CHIKV. The detection of ZIKV was further enhanced with NT particles by processing of larger volume urine samples. Furthermore, we developed a magnetic NT particle, CN3080, based on the same backbone of CN1030, and demonstrated that CN3080 could also capture and enrich ZIKV and CHIKV in a dose-dependent manner. Finally, in silico docking predictions support that the affinity between reactive red 120 and ZIKV or CHIKV envelope proteins appeared to be greater than acrylic acid. Overall, our data show that NT particles along with reactive red 120 can be utilized as a pre-processing technology for enhancement of detecting febrile-illness causing viruses.
Conflict of interest statement
Authors AP and BL are employed by the company Ceres Nanosciences, Inc. Author KKH is a member of the Scientific Advisory board at Ceres Nanosciences, Inc. BL is a shareholder at Ceres Nanosciences, Inc. The nanoparticles used in this study were research grade and provided by Ceres Nanosciences, Inc which are not commercially available products. This does not alter our adherence to PLOS ONE policies on sharing data and materials. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

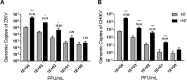
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

