Significance of the gut microbiome in multistep colorectal carcinogenesis
- PMID: 31910311
- PMCID: PMC7060472
- DOI: 10.1111/cas.14298
Significance of the gut microbiome in multistep colorectal carcinogenesis
Abstract
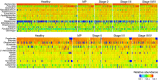
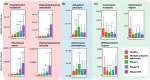
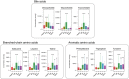
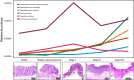
Colorectal cancer (CRC) is highly prevalent worldwide. In 2018, there were over 1.8 million new cases. Most sporadic CRC develop from polypoid adenomas and are preceded by intramucosal carcinoma (stage 0), which can progress into more malignant forms. This developmental process is known as the adenoma-carcinoma sequence. Early detection and endoscopic removal are crucial for CRC management. Accumulating evidence suggests that the gut microbiota is associated with CRC development in humans. Comprehensive characterization of this microbiota is of great importance to assess its potential as a diagnostic marker in the very early stages of CRC. In this review, we summarized recent studies on CRC-associated bacteria and their carcinogenic mechanisms in animal models, human cell lines and human cohorts. High-throughput technologies have facilitated the identification of CRC-associated bacteria in human samples. We have presented our metagenome and metabolome studies on fecal samples collected from a large Japanese cohort that revealed stage-specific phenotypes of the microbiota in CRC. Furthermore, we have discussed the potential carcinogenic mechanisms of the gut microbiota, from which we can infer whether changes in the gut microbiota are a cause or effect in the multi-step process of CRC carcinogenesis.
Keywords: adenoma-carcinoma sequence; colorectal cancer; gut microbiome; metabolome; metagenome.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Takuji Yamada is a founder of Metabologenomics. The company is focused on the design and control of the gut environment for human health. The company has no control over the interpretation, writing or publication of this work. The terms of these arrangements are being managed by the Tokyo Institute of Technology in accordance with its conflict of interest policies.
Figures

References
-
- Kolonel LN, Altshuler D, Henderson BE. The multiethnic cohort study: exploring genes, lifestyle and cancer risk. Nat Rev Cancer. 2004;4:519‐527. - PubMed
-
- Kuriki K, Tajima K. The increasing incidence of colorectal cancer and the preventive strategy in Japan. Asian Pac J Cancer Prev. 2006;7:495‐501. - PubMed
-
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759‐767. - PubMed
Publication types
MeSH terms
Grants and funding
- JPMJCR19U3/Japan Science and Technology Agency
- JP18ek0109187/Japan Agency for Medical Research and Development
- JP18jk0210009/Japan Agency for Medical Research and Development
- JP19cm0106464/Japan Agency for Medical Research and Development
- 25-A-4/National Cancer Center Research and Development Fund
- 28-A-4/National Cancer Center Research and Development Fund
- 29-A-6/National Cancer Center Research and Development Fund
- Joint Research Project of the Institute of Medical Science, the University of Tokyo
- JPMJPR1507/Precursory Research for Embryonic Science and Technology
- the Takeda Science Foundation
- 142558/Japan Society for the Promotion of Science
- 16J10135/Japan Society for the Promotion of Science
- 221S0002/Japan Society for the Promotion of Science
- the Yasuda Medical Foundation
- the Yakult Bio-Science Foundation
- the Princess Takamatsu Cancer Research Fund
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

