Synthesis, in vitro screening and molecular docking of isoquinolinium-5-carbaldoximes as acetylcholinesterase and butyrylcholinesterase reactivators
- PMID: 31910701
- PMCID: PMC6968506
- DOI: 10.1080/14756366.2019.1710501
Synthesis, in vitro screening and molecular docking of isoquinolinium-5-carbaldoximes as acetylcholinesterase and butyrylcholinesterase reactivators
Abstract
The series of symmetrical and unsymmetrical isoquinolinium-5-carbaldoximes was designed and prepared for cholinesterase reactivation purposes. The novel compounds were evaluated for intrinsic acetylcholinesterase (AChE) or butyrylcholinesterase (BChE) inhibition, when the majority of novel compounds resulted with high inhibition of both enzymes and only weak inhibitors were selected for reactivation experiments on human AChE or BChE inhibited by sarin, VX, or paraoxon. The AChE reactivation for all used organophosphates was found negligible if compared to the reactivation ability of obidoxime. Importantly, two compounds were found to reactivate BChE inhibited by sarin or VX better to obidoxime at human attainable concentration. One compound resulted as better reactivator of NEMP (VX surrogate)-inhibited BChE than obidoxime. The in vitro results were further rationalized by molecular docking studies showing future directions on designing potent BChE reactivators.
Keywords: Acetylcholinesterase; butyrylcholinesterase; organophosphate; oxime; reactivator.
Conflict of interest statement
The authors report no conflicts of interest.
Figures






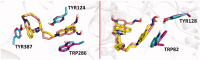


References
-
- Starke K. Presynaptic receptors. Annu Rev Pharmacol Toxicol 1981;21:7–30. - PubMed
-
- Dolezal R, Korabecny J, Malinak D, et al. . Ligand-based 3D QSAR analysis of reactivation potency of mono-and bis-pyridinium aldoximes toward VX-inhibited rat acetylcholinesterase. J Mol Graph 2015;56:113–29. - PubMed
-
- Musilek K, Dolezal M, Gunn‐Moore F, et al. . Design, evaluation and structure—Activity relationship studies of the AChE reactivators against organophosphorus pesticides. Med Res Rev 2011;31:548–75. - PubMed
-
- Watson A, Opresko D, Young RA, et al. . Organophosphate nerve agents. Handbook of toxicology of chemical warfare agents. 2nd ed. London: Handbook of Toxicology of Chemical Warfare Agents; 2009.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
