Outcomes of Adult Heart Transplantation Using Hepatitis C-Positive Donors
- PMID: 31910781
- PMCID: PMC7033844
- DOI: 10.1161/JAHA.119.014495
Outcomes of Adult Heart Transplantation Using Hepatitis C-Positive Donors
Abstract
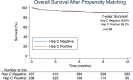
Background This study evaluated the impact of hepatitis C-positive (HCV+) donors on outcomes of heart transplantation in the United States. Methods and Results Adults undergoing isolated heart transplantation in the United States between January 1, 2016, and December 31, 2018, were included. The primary outcome was 1-year post-transplant survival. Multivariable Cox regression and 2:1 propensity matching were used to compare outcomes between transplants with HCV+ and hepatitis C-negative (HCV-) donors. A subanalysis was performed to evaluate the impact of nucleic acid amplification test positivity on outcomes. Of 7889 isolated heart transplants performed during the study period, 343 (4.4%) used HCV+ donors. Overall unadjusted 1-year posttransplant survival was not statistically different between HCV- versus HCV+ donors (91.1% versus 90.2%; P=0.86), a finding that persisted after risk adjustment (hazard ratio, 1.05; 95% CI, 0.70-1.58; P=0.80). Propensity matching resulted in 675 well-balanced patients (437 HCV- and 238 HCV+). Overall 1-year posttransplant survival was not statistically different in propensity-matched analysis (89.8% HCV- versus 89.2% HCV+; P=0.88). Rates of 1-year drug-treated rejection (21.1% versus 22.1%; P=0.84), postoperative dialysis (11.4% versus 14.7%; P=0.22), and stroke (4.6% versus 2.1%; P=0.10) were also not statistically different between HCV- and HCV+ groups, respectively. Outcomes were not statistically different between nucleic acid amplification test-negative and nucleic acid amplification test-positive HCV+ donors. Conclusions Adult heart transplants using HCV+ donors, including those that are nucleic acid amplification test positive, can be performed without an adverse impact on 1-year survival. Wider implementation of protocols for using HCV+ donors and an assessment of longer-term outcomes including seroconversion rates will be important in maximizing the effect of HCV+ donors on national donor shortages.
Keywords: heart failure; heart transplantation; hepatitis C; rejection.
Figures
References
-
- Gasink LB, Blumberg EA, Localio AR, Desai SS, Israni AK, Lautenbach E. Hepatitis C virus seropositivity in organ donors and survival in heart transplant recipients. JAMA. 2006;296:1843–1850. - PubMed
-
- Woolley AE, Singh SK, Goldberg HJ, Mallidi HR, Givertz MM, Mehra MR, Coppolino A, Kusztos AE, Johnson ME, Chen K, Haddad EA, Fanikos J, Harrington DP, Camp PC, Baden LR; DONATE HCV Trial Team . Heart and lung transplants from HCV‐infected donors to uninfected recipients. N Engl J Med. 2019;380:1606–1617. - PMC - PubMed
-
- Schlendorf KH, Zalawadiya S, Shah AS, Wigger M, Chung CY, Smith S, Danter M, Choi CW, Keebler ME, Brinkley DM, Sacks SB, Ooi H, Perri R, Awad JA, Lewis S, Hayes R, O'Dell H, Darragh C, Carver A, Edmonds C, Ruzevich‐Scholl S, Lindenfeld J. Early outcomes using hepatitis C–positive donors for cardiac transplantation in the era of effective direct‐acting anti‐viral therapies. J Heart Lung Transplant. 2018;37:763–769. - PubMed
-
- McLean RC, Reese PP, Acker M, Atluri P, Bermudez C, Goldberg LR, Abt PL, Blumberg EA, Van Deerlin VM, Reddy KR, Bloom RD, Hasz R, Suplee L, Sicilia A, Woodards A, Zahid MN, Bar KJ, Porrett P, Levine MH, Hornsby N, Gentile C, Smith J, Goldberg DS. Transplanting hepatitis C virus–infected hearts into uninfected recipients: a single‐arm trial. Am J Transplant. 2019;19:2533–2542. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical