Long-Term Neurobehavioral and Quality of Life Outcomes of Critically Ill Children after Glycemic Control
- PMID: 31910992
- PMCID: PMC7122648
- DOI: 10.1016/j.jpeds.2019.10.055
Long-Term Neurobehavioral and Quality of Life Outcomes of Critically Ill Children after Glycemic Control
Abstract
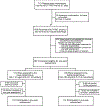
Objectives: To investigate adaptive skills, behavior, and quality health-related quality of life in children from 32 centers enrolling in the Heart And Lung Failure-Pediatric INsulin Titration randomized controlled trial.
Study design: This prospective longitudinal cohort study compared the effect of 2 tight glycemic control ranges (lower target, 80-100 mg/dL vs higher target, 150-180 mg/dL) 1-year neurobehavioral and health-related quality of life outcomes. Subjects had confirmed hyperglycemia and cardiac and/or respiratory failure. Patients aged 2-16 years old enrolled between April 2012 and September 2016 were studied at 1 year after intensive care discharge. The primary outcome, adaptive skills, was assessed using the Vineland Adaptive Behavior Scale. Behavior and health-related quality of life outcomes were assessed as secondary outcomes using the Pediatric Quality of Life and Child Behavior Checklist at baseline and 1-year follow-up. Group differences were evaluated using regression models adjusting for age category, baseline overall performance, and risk of mortality.
Results: Of 369 eligible children, 358 survived after hospital discharge and 214 (60%) completed follow-up. One-year Vineland Adaptive Behavior Scale-II composite scores were not different (mean ± SD, 79.9 ± 25.5 vs 79.4 ± 26.9, lower vs higher target; P = .20). Improvement in Pediatric Quality of Life total health from baseline was greater in the higher target group (adjusted mean difference, 8.2; 95% CI, 1.1-15.3; P = .02).
Conclusions: One-year adaptive behavior in critically ill children with lower vs higher target glycemic control did not differ. The higher target group demonstrated improvement from baseline in overall health. This study affirms the lack of benefit of lower glucose targeting.
Trial registration: ClinicalTrials.gov: NCT01565941.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interests.
Figures
References
-
- Van Den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001; 345:1359–67. - PubMed
-
- Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in medical ICU. N Engl J Med. 2006; 354:449–61. - PubMed
-
- Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, et al. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009; 373:547–56. - PubMed
-
- Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008; 358:125–39. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

