The drug adaptaquin blocks ATF4/CHOP-dependent pro-death Trib3 induction and protects in cellular and mouse models of Parkinson's disease
- PMID: 31911115
- PMCID: PMC7545957
- DOI: 10.1016/j.nbd.2019.104725
The drug adaptaquin blocks ATF4/CHOP-dependent pro-death Trib3 induction and protects in cellular and mouse models of Parkinson's disease
Abstract
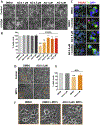
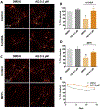
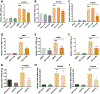
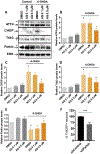
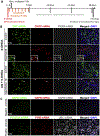
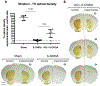
Identifying disease-causing pathways and drugs that target them in Parkinson's disease (PD) has remained challenging. We uncovered a PD-relevant pathway in which the stress-regulated heterodimeric transcription complex CHOP/ATF4 induces the neuron prodeath protein Trib3 that in turn depletes the neuronal survival protein Parkin. Here we sought to determine whether the drug adaptaquin, which inhibits ATF4-dependent transcription, could suppress Trib3 induction and neuronal death in cellular and animal models of PD. Neuronal PC12 cells and ventral midbrain dopaminergic neurons were assessed in vitro for survival, transcription factor levels and Trib3 or Parkin expression after exposure to 6-hydroxydopamine or 1-methyl-4-phenylpyridinium with or without adaptaquin co-treatment. 6-hydroxydopamine injection into the medial forebrain bundle was used to examine the effects of systemic adaptaquin on signaling, substantia nigra dopaminergic neuron survival and striatal projections as well as motor behavior. In both culture and animal models, adaptaquin suppressed elevation of ATF4 and/or CHOP and induction of Trib3 in response to 1-methyl-4-phenylpyridinium and/or 6-hydroxydopamine. In culture, adaptaquin preserved Parkin levels, provided neuroprotection and preserved morphology. In the mouse model, adaptaquin treatment enhanced survival of dopaminergic neurons and substantially protected their striatal projections. It also significantly enhanced retention of nigrostriatal function. These findings define a novel pharmacological approach involving the drug adaptaquin, a selective modulator of hypoxic adaptation, for suppressing Parkin loss and neurodegeneration in toxin models of PD. As adaptaquin possesses an oxyquinoline backbone with known safety in humans, these findings provide a firm rationale for advancing it towards clinical evaluation in PD.
Keywords: ATF4; Adaptaquin; CHOP; Neuroprotection; Oxyquinoline; Parkin; Parkinson's disease; Trib3.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Aimé P, Sun X, Greene LA, 2018. Manipulation and study of gene expression in neurotoxin-treated PC12 and SH-SY5Y cells for in vitro studies of Parkinson’s disease gene expression and regulation in mammalian cells. In: Transcription Toward the Establishment of Novel Therapeutics. IntechOpen 2018. 10.5772/intechopen.71811. - DOI
-
- Bowenkamp KE, et al., 1996. 6-hydroxydopamine induces the loss of the dopaminergic phenotype in substantia nigra neurons of the rat. A possible mechanism for restoration of the nigrostriatal circuit mediated by glial cell line-derived neurotrophic factor. Exp. Brain Res 111, 1–7. 10.1007/bf00229549. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

