Regulatory T-cell Depletion Alters the Tumor Microenvironment and Accelerates Pancreatic Carcinogenesis
- PMID: 31911451
- PMCID: PMC7224338
- DOI: 10.1158/2159-8290.CD-19-0958
Regulatory T-cell Depletion Alters the Tumor Microenvironment and Accelerates Pancreatic Carcinogenesis
Abstract
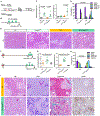
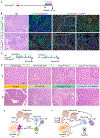
Regulatory T cells (Treg) are abundant in human and mouse pancreatic cancer. To understand the contribution to the immunosuppressive microenvironment, we depleted Tregs in a mouse model of pancreatic cancer. Contrary to our expectations, Treg depletion failed to relieve immunosuppression and led to accelerated tumor progression. We show that Tregs are a key source of TGFβ ligands and, accordingly, their depletion reprogramed the fibroblast population, with loss of tumor-restraining, smooth muscle actin-expressing fibroblasts. Conversely, we observed an increase in chemokines Ccl3, Ccl6, and Ccl8 leading to increased myeloid cell recruitment, restoration of immune suppression, and promotion of carcinogenesis, an effect that was inhibited by blockade of the common CCL3/6/8 receptor CCR1. Further, Treg depletion unleashed pathologic CD4+ T-cell responses. Our data point to new mechanisms regulating fibroblast differentiation in pancreatic cancer and support the notion that fibroblasts are a heterogeneous population with different and opposing functions in pancreatic carcinogenesis. SIGNIFICANCE: Here, we describe an unexpected cross-talk between Tregs and fibroblasts in pancreatic cancer. Treg depletion resulted in differentiation of inflammatory fibroblast subsets, in turn driving infiltration of myeloid cells through CCR1, thus uncovering a potentially new therapeutic approach to relieve immunosuppression in pancreatic cancer.See related commentary by Aykut et al., p. 345.This article is highlighted in the In This Issue feature, p. 327.
©2020 American Association for Cancer Research.
Conflict of interest statement
Figures





Comment in
-
Regulatory T Cells Keep Pancreatic Cancer at Bay.Cancer Discov. 2020 Mar;10(3):345-347. doi: 10.1158/2159-8290.CD-20-0002. Cancer Discov. 2020. PMID: 32127405
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA198074/CA/NCI NIH HHS/United States
- P30 CA046592/CA/NCI NIH HHS/United States
- F32 CA228328/CA/NCI NIH HHS/United States
- T32 GM145304/GM/NIGMS NIH HHS/United States
- T32 GM113900/GM/NIGMS NIH HHS/United States
- R01 CA151588/CA/NCI NIH HHS/United States
- K08 CA201581/CA/NCI NIH HHS/United States
- U01 CA224145/CA/NCI NIH HHS/United States
- L30 CA188846/CA/NCI NIH HHS/United States
- T32 GM007315/GM/NIGMS NIH HHS/United States
- R25 GM086262/GM/NIGMS NIH HHS/United States
- T32 CA009676/CA/NCI NIH HHS/United States
- P30 DK034933/DK/NIDDK NIH HHS/United States
- T32 DK094775/DK/NIDDK NIH HHS/United States
- R50 CA232985/CA/NCI NIH HHS/United States
- R37 CA237421/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

