Matrix Redox Physiology Governs the Regulation of Plant Mitochondrial Metabolism through Posttranslational Protein Modifications
- PMID: 31911454
- PMCID: PMC7054041
- DOI: 10.1105/tpc.19.00535
Matrix Redox Physiology Governs the Regulation of Plant Mitochondrial Metabolism through Posttranslational Protein Modifications
Abstract
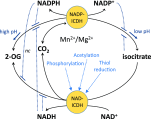
Mitochondria function as hubs of plant metabolism. Oxidative phosphorylation produces ATP, but it is also a central high-capacity electron sink required by many metabolic pathways that must be flexibly coordinated and integrated. Here, we review the crucial roles of redox-associated posttranslational protein modifications (PTMs) in mitochondrial metabolic regulation. We discuss several major concepts. First, the major redox couples in the mitochondrial matrix (NAD, NADP, thioredoxin, glutathione, and ascorbate) are in kinetic steady state rather than thermodynamic equilibrium. Second, targeted proteomics have produced long lists of proteins potentially regulated by Cys oxidation/thioredoxin, Met-SO formation, phosphorylation, or Lys acetylation, but we currently only understand the functional importance of a few of these PTMs. Some site modifications may represent molecular noise caused by spurious reactions. Third, different PTMs on the same protein or on different proteins in the same metabolic pathway can interact to fine-tune metabolic regulation. Fourth, PTMs take part in the repair of stress-induced damage (e.g., by reducing Met and Cys oxidation products) as well as adjusting metabolic functions in response to environmental variation, such as changes in light irradiance or oxygen availability. Finally, PTMs form a multidimensional regulatory system that provides the speed and flexibility needed for mitochondrial coordination far beyond that provided by changes in nuclear gene expression alone.
© 2020 American Society of Plant Biologists. All rights reserved.
Figures



References
-
- Agius S.C., Rasmusson A.G., Møller I.M. (2001). NAD(P) turnover in plant mitochondria. Aust. J. Plant Physiol. 28: 461–470.
-
- Albrecht S.C., Sobotta M.C., Bausewein D., Aller I., Hell R., Dick T.P., Meyer A.J. (2014). Redesign of genetically encoded biosensors for monitoring mitochondrial redox status in a broad range of model eukaryotes. J. Biomol. Screen. 19: 379–386. - PubMed
-
- Atkin O.K., Evans J.R., Siebke K. (1998). Relationship between the inhibition of leaf respiration by light and enhancement of leaf dark respiration following light treatment. Aust. J. Plant Physiol. 25: 437–443.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

