Asthma control and COPD symptom burden in patients using fixed-dose combination inhalers (SPRINT study)
- PMID: 31911607
- PMCID: PMC6946676
- DOI: 10.1038/s41533-019-0159-1
Asthma control and COPD symptom burden in patients using fixed-dose combination inhalers (SPRINT study)
Abstract
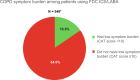
Previous studies have found suboptimal control of symptom burden to be widespread among patients with asthma and chronic obstructive pulmonary disease (COPD). The Phase IV SPRINT study was conducted in 10 countries in Europe to assess asthma disease control and COPD symptom burden in patients treated with a fixed-dose combination (FDC) of inhaled corticosteroids (ICS) and long-acting beta agonists (LABAs). SPRINT included 1101 patients with asthma and 560 with COPD; all were receiving treatment with an FDC of ICS/LABA, delivered via various inhalers. Data were obtained over a 3-month period, during a single routine physician's office visit. Asthma control was defined as Asthma Control Test (ACT) score >19. COPD symptom burden was assessed by COPD Assessment Test (CAT), with a CAT score <10 defining low COPD symptom burden. Among patients using any ICS/LABA FDC, 62% of patients with asthma had achieved disease control (ACT score >19) and 16% of patients with COPD had low symptom burden (CAT score <10).
Conflict of interest statement
N.R. reports grants and personal fees from Boehringer Ingelheim, and Novartis and personal fees from Teva, GSK, AstraZeneca, Chiesi, Mundipharma, Cipla, Sanofi, Sandoz, 3M, Pfizer, and Zambon outside of the published work. V.B., I.C., J.v.d.P., and C.G. declare no conflicts of interest. D.S. received grants and/or personal fees from AstraZeneca, Apellis, Boehringer Ingelheim, Chiesi, Cipla, Genetech, GlaxoSmithKline, Glenmark, Menarini, Merck, Mundipharma, Novartis, Peptinnovate, Pfizer, Pulmatrix, Skyepharma, Teva, Theravance, and Verona. V.P. in the last three years has received honoraria for speaking at sponsored meetings from Astrazeneca, Chiesi, and Novartis; received help assistance to meeting travel from Astrazeneca, Chiesi, and Novartis; acts as a consultant for ALK, Astrazeneca, Boehringer, MundiPharma, and Sanofi; and has received funding/grant support for research projects from a variety of Government agencies and not-for-profit foundations, as well as AstraZeneca, Chiesi, and Menarini. G.S. and O.P. are employees of Teva Pharmaceuticals. I.S. was an employee of Teva Pharmaceuticals at the time the study was conducted.
Figures

References
-
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017;5:691–706. doi: 10.1016/S2213-2600(17)30293-X. - DOI - PMC - PubMed
-
- Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018 GINA report. https://ginasthma.org/wp-content/uploads/2019/01/2018-GINA.pdf (2018). Accessed 11 Oct 2019.
-
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2018 Report. http://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revi... (2018). Accessed 11 Oct 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

