SPOP targets oncogenic protein ZBTB3 for destruction to suppress endometrial cancer
- PMID: 31911863
- PMCID: PMC6943363
SPOP targets oncogenic protein ZBTB3 for destruction to suppress endometrial cancer
Erratum in
-
Erratum: SPOP targets oncogenic protein ZBTB3 for destruction to suppress endometrial cancer.Am J Cancer Res. 2021 Apr 15;11(4):1792-1794. eCollection 2021. Am J Cancer Res. 2021. PMID: 33948389 Free PMC article.
-
Erratum: SPOP targets oncogenic protein ZBTB3 for destruction to suppress endometrial cancer.Am J Cancer Res. 2023 Nov 15;13(11):5748-5749. eCollection 2023. Am J Cancer Res. 2023. PMID: 38058828 Free PMC article.
-
Erratum: SPOP targets oncogenic protein ZBTB3 for destruction to suppress endometrial cancer.Am J Cancer Res. 2025 Jul 25;15(7):3332-3337. doi: 10.62347/IVFL8331. eCollection 2025. Am J Cancer Res. 2025. PMID: 40814367 Free PMC article.
Abstract
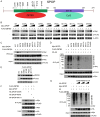
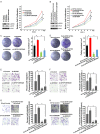
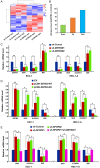
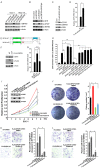
Dysregulation of the ubiquitin-proteasome pathway is closely associated with cancer initiation and progression. SPOP is an adapter protein of the CUL3-based E3 ubiquitin ligase complexes. Several whole genome/exome sequencing studies on endometrial cancers (ECs) revealed that the SPOP gene is frequently mutated. However, how SPOP mutations contribute to EC remains poorly understood. In this study, transcription factor ZBTB3 was identified as a proteolytic substrate for the SPOP-CUL3-RBX1 E3 ubiquitin ligase complex. SPOP specifically recognizes two Ser/Thr (S/T)-rich degrons located in ZBTB3 and triggers the degradation of ZBTB3 via the ubiquitin-proteasome pathway. By contrast, EC-associated SPOP mutants are defective in regulating ZBTB3 stability. SPOP inactivation promotes endometrial cell proliferation, migration, and invasion partly through ZBTB3 accumulation. Sonic hedgehog (SHH) was found to be a transcriptional target of ZBTB3. SPOP inactivation leads to ZBTB3-dependent SHH upregulation in EC cells. RUSKI-43, a small molecule inhibitor of SHH, suppresses cell proliferation, migration, and invasion in SPOP-depleted or EC-associated SPOP mutant-overexpressed EC cells. Our data indicate that pharmacological inhibition of SHH represents a possible treatment strategy for SPOP-mutated ECs.
Keywords: SPOP; ZBTB3; endometrial cancer; sonic hedgehog; ubiquitination.
AJCR Copyright © 2019.
Conflict of interest statement
None.
Figures



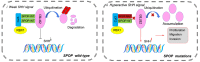
Similar articles
-
Endometrial cancer-associated mutants of SPOP are defective in regulating estrogen receptor-α protein turnover.Cell Death Dis. 2015 Mar 12;6(3):e1687. doi: 10.1038/cddis.2015.47. Cell Death Dis. 2015. PMID: 25766326 Free PMC article.
-
SPOP promotes ATF2 ubiquitination and degradation to suppress prostate cancer progression.J Exp Clin Cancer Res. 2018 Jul 11;37(1):145. doi: 10.1186/s13046-018-0809-0. J Exp Clin Cancer Res. 2018. PMID: 29996942 Free PMC article.
-
SPOP-dependent destabilization of SYT12 in a GSK-3β-dependent manner in papillary thyroid cancer cells.Clin Exp Med. 2025 Jul 10;25(1):243. doi: 10.1007/s10238-025-01791-z. Clin Exp Med. 2025. PMID: 40640517 Free PMC article.
-
Electronic cigarettes for smoking cessation and reduction.Cochrane Database Syst Rev. 2014;(12):CD010216. doi: 10.1002/14651858.CD010216.pub2. Epub 2014 Dec 17. Cochrane Database Syst Rev. 2014. PMID: 25515689
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
Cited by
-
Prostate cancer-associated SPOP mutations lead to genomic instability through disruption of the SPOP-HIPK2 axis.Nucleic Acids Res. 2021 Jul 9;49(12):6788-6803. doi: 10.1093/nar/gkab489. Nucleic Acids Res. 2021. PMID: 34133717 Free PMC article.
-
Challenges and opportunities for the diverse substrates of SPOP E3 ubiquitin ligase in cancer.Theranostics. 2025 May 8;15(13):6111-6145. doi: 10.7150/thno.113356. eCollection 2025. Theranostics. 2025. PMID: 40521202 Free PMC article. Review.
-
LZTR1: A promising adaptor of the CUL3 family.Oncol Lett. 2021 Jul;22(1):564. doi: 10.3892/ol.2021.12825. Epub 2021 May 29. Oncol Lett. 2021. PMID: 34113392 Free PMC article. Review.
-
SPOP/NOLC1/B4GALT1 signaling axis enhances paclitaxel resistance in endometrial cancer by inducing O-dysglycosylation.Oncogene. 2025 Jun;44(22):1694-1708. doi: 10.1038/s41388-025-03347-7. Epub 2025 Mar 17. Oncogene. 2025. PMID: 40097806
-
Regulator of cullins-1 (ROC1) negatively regulates the Gli2 regulator SUFU to activate the hedgehog pathway in bladder cancer.Cancer Cell Int. 2021 Jan 26;21(1):75. doi: 10.1186/s12935-021-01775-5. Cancer Cell Int. 2021. PMID: 33499884 Free PMC article.
References
-
- Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International patterns and trends in endometrial cancer incidence, 1978-2013. J Natl Cancer Inst. 2018;110:354–361. - PubMed
-
- Sutton G, Axelrod JH, Bundy BN, Roy T, Homesley HD, Malfetano JH, Mychalczak BR, King ME. Whole abdominal radiotherapy in the adjuvant treatment of patients with stage III and IV endometrial cancer: a gynecologic oncology group study. Gynecol Oncol. 2005;97:755–763. - PubMed
-
- Le Gallo M, O’Hara AJ, Rudd ML, Urick ME, Hansen NF, O’Neil NJ, Price JC, Zhang S, England BM, Godwin AK, Sgroi DC NIH Intramural Sequencing Center (NISC) Comparative Sequencing Program. Hieter P, Mullikin JC, Merino MJ, Bell DW. Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nat Genet. 2012;44:1310–1315. - PMC - PubMed
-
- Cancer Genome Atlas Research Network. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, Laird PW, Ding L, Zhang W, Mills GB, Kucherlapati R, Mardis ER, Levine DA. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. - PMC - PubMed
-
- Zhao S, Choi M, Overton JD, Bellone S, Roque DM, Cocco E, Guzzo F, English DP, Varughese J, Gasparrini S, Bortolomai I, Buza N, Hui P, Abu-Khalaf M, Ravaggi A, Bignotti E, Bandiera E, Romani C, Todeschini P, Tassi R, Zanotti L, Carrara L, Pecorelli S, Silasi DA, Ratner E, Azodi M, Schwartz PE, Rutherford TJ, Stiegler AL, Mane S, Boggon TJ, Schlessinger J, Lifton RP, Santin AD. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc Natl Acad Sci U S A. 2013;110:2916–2921. - PMC - PubMed
LinkOut - more resources
Full Text Sources
