Shockwave Therapy Efficiently Cures Multispecies Chronic Periodontitis in a Humanized Rat Model
- PMID: 31911896
- PMCID: PMC6923175
- DOI: 10.3389/fbioe.2019.00382
Shockwave Therapy Efficiently Cures Multispecies Chronic Periodontitis in a Humanized Rat Model
Abstract
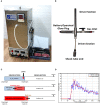
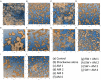
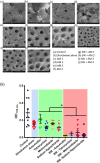
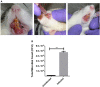
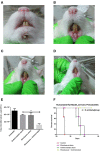
Biofilms are ubiquitous in nature and are invariably associated with health and diseases of all living beings. Periodontal diseases & dental caries are the most prevalent conditions in which biofilm has established as a primary causative factor. Managing poly-microbial biofilm is the mainstay of periodontal therapy. Plethora of antimicrobials have been used till date to combat biofilm, but the emergence of antibiotic tolerance and resistance in biofilms is a major cause of concern. Apart from use of antimicrobials, various anti-biofilm strategies have evolved which include the use of mechanical, and chemical means to disrupt biofilms. However, none of these approaches have led to desired or optimal biofilm control and hence search for novel approach continues. Shockwaves are used in medical practice for various therapeutic purposes and in local drug delivery, gene therapy, wound healing & regeneration. With this background, a study was designed with an attempt to explore the possibility of using the shockwave for their effect on multispecies oral biofilm development from subgingival plaque samples obtained from chronic periodontitis patients. Plaque samples from 25 patients were used to derive multispecies biofilm which were used to check the efficacy of shockwaves and antibacterial efficacy of four clinically relevant antimicrobials. Biofilms were analyzed by scanning electron microscope; atomic force microscope and their biomass was quantitated by crystal violet staining. Further, a humanized rat model of periodontitis was developed. Patient derived plaque was used to establish periodontitis in healthy rats. The model was validated by performing colony forming unit (CFU) analysis of the infected tissue. The animals were subjected to low intensity shockwaves using a hand-held shockwave generator at the site of infection. Shockwave treatment was done with or without antimicrobial application. The animals were monitored for clearance of infection and for mortality. The results show that shockwave treatment in combination with antimicrobials is significantly effective in clearing a multispecies biofilm. This also brings out the possibility of application of shockwaves in the management of oral biofilms either alone or in combination with established antimicrobial agents. With further research, safety profile validation and clinical trials, shockwaves can be an effective, novel approach in management of biofilm associated periodontal disease.
Keywords: biofilms; humanized rat model; patient sample; periodontitis; shock waves.
Copyright © 2019 Datey, Thaha, Patil, Gopalan and Chakravortty.
Figures

References
-
- Atkinson B., Fowler H. W. (1974). The significance of microbial film in fermenters, in Advances in Biochemical Engineering (Berlin; Heidelberg: Springer; ), 10.1007/3-540-06546-6_7 - DOI
-
- Cerca N., Silvia Martins F. C., Jefferson K. K., Pier G. B., Oliveira R., Azeredo J. (2005). Comparative assessment of antibiotic susceptibility of coagulase-negative staphylococci in biofilm versus planktonic culture as assessed by bacterial enumeration or rapid XTT colorimetry. J. Antimicrob. Chemother. 56, 331–336. 10.1093/jac/dki217 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

