A French Value Set for the EQ-5D-5L
- PMID: 31912325
- PMCID: PMC7080328
- DOI: 10.1007/s40273-019-00876-4
A French Value Set for the EQ-5D-5L
Abstract
Objective: The objective of this study was to develop a French value set for the EQ-5D-5L, for academic and clinical research, and for regulatory requirements for price-setting of drugs and medical devices.
Method: This study used the standardized valuation protocol developed by EuroQol, using computer-assisted personal interview software. A representative sample of 1048 French residents were interviewed by a market research company, under the supervision of the research team. Health states were valued using composite time trade-off and a discrete choice experiment. Modeling was used to create values for the 3125 possible health states. The composite time trade-off data were modeled using a Tobit model with censored observations at -1 and correcting for heteroscedasticity. A conditional logit model was used for the discrete choice results, and both models were combined using a hybrid model. An adjusted hybrid model was tested to correct for imbalance in the sample on age and sex compared with the general population. A comparison with the 3-level (3L) value set was performed.
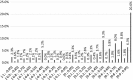
Results: The adjusted model was preferred to comply with the representativeness of the general population. It provided a value set for which all coefficients were logically consistent. Values ranged from - 0.525 to 1. The distribution of values presented a shift towards higher values versus the 3L value set. Ranking of dimensions changed. Pain and discomfort and mobility were the dimensions with the highest potential for disutility compared with mobility and self-care for the 3L instrument.
Conclusions: This study provides a value set based on societal preferences of the French population, using an improved descriptive instrument of health-related quality-of-life health states. It will contribute to improve the quality of cost-effectiveness analysis in the French context and help stimulate disease-specific quality-of-life references for academic-, institutional-, and industry-promoted studies.
Conflict of interest statement
Gérard de Pouvourville was Full Professor at the ESSEC Business School at the time of this study. He has been, and is, a regular consultant for the funding companies, as a member of advisory boards on cost-effectiveness analyses. The ESSEC has also received funding to perform cost-effectiveness analysis. None of the existing funding has been related to developing value sets for quality-of-life questionnaires. The ESSEC is currently funded to perform a survey on the quality of life of French inflammatory bowel disease patients using EQ-5D-5L. The funding sources were Euroqol, Amgen, MSD, Janssen Cilag and Biogaran, through unrestricted research grants. Luiz Andrade was a research assistant at the time of the study and was employed by the ESSEC. He has no direct financial conflicts of interest. Kristina Ludwig worked as a freelancer for the EuroQol Research Foundation during the data collection phase of the study and has received research grants from the Foundation to conduct methodological research. She has received no direct payment from industrial sponsors for this study. As a collaborator of EuroQol and the main investigator of the German Value Set, she supports the EQ-5D-5L and the methodological choices made by the Foundation. Mark Oppe was an employee of the EuroQol Research Foundation at the time of the study, but now works for Axentiva Solutions, a consulting company based in Spain. He was a main contributor to the development of the EQ-5D-5L valuation methodology, specifically for the cTTO protocol, the Quality Control module, and the development of the hybrid methodology. Juan Manuel Ramos Goni is a member of EuroQol and founder of Axentiva Solutions. He has received no direct payment from industry sponsors for the French study. He also contributed to the development of the EQ-5D-5L valuation methodology, specifically for the development of the hybrid methodology.
Figures





References
-
- Decree n° 2012-1116, October 2, 2012, defining the health economics missions of the High Health Authority. French Republic Offical Gazette n° 0231, October 4 2012, page 15222, text n° 8. https://www.legifrance.gouv.fr/.
-
- Decision n°2013.0111/DC/SEESP, September 18, 2013: Collge of the High Health Authority relative to the significiant impact on expenditures for the National Sickness Fund triggering the need for an economic assessment of health products claiming an improvement/expected of Medical Serivce Rendered of I, II or III. https://www.has-sante.fr/portail/upload/docs/application/pdf/2013-09/c_2....
-
- Haute Autorité de Santé (HAS). Choices in methods for economic evaluation. Department of Economics and Public Health Assessment. Haute Autorité de Santé; Oct 2012. https://www.has-sante.fr/portail/upload/docs/application/pdf/2012-10/cho....
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

