Differential effects of an electronic symptom monitoring intervention based on the age of patients with advanced cancer
- PMID: 31912785
- PMCID: PMC7497788
- DOI: 10.1016/j.annonc.2019.09.003
Differential effects of an electronic symptom monitoring intervention based on the age of patients with advanced cancer
Abstract
Background: Symptom monitoring interventions enhance patient outcomes, including quality of life (QoL), health care utilization, and survival, but it remains unclear whether older and younger patients with cancer derive similar benefits. We explored whether age moderates the improved outcomes seen with an outpatient electronic symptom monitoring intervention.
Patients and methods: We carried out a secondary analysis of data from a randomized trial of 766 patients receiving chemotherapy for metastatic solid tumors. Patients received an electronic symptom monitoring intervention integrated with oncology care or usual oncology care alone. The intervention consisted of patients reporting their symptoms, which were provided to their physicians at clinic visits, and nurses receiving alerts for severe/worsening symptoms. We used regression models to determine whether age (older or younger than 70 years) moderated the effects of the intervention on QoL (EuroQol EQ-5D), emergency room (ER) visits, hospitalizations, and survival outcomes.
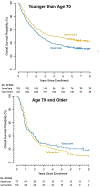
Results: Enrollment rates for younger (589/777 = 75.8%) and older (177/230 = 77.0%) patients did not differ. Older patients (median age = 75 years, range 70-91 years) were more likely to have an education level of high school or less (26.6% versus 20.9%, P = 0.029) and to be computer inexperienced (50.3% versus 23.4%, P < 0.001) compared with younger patients (median age = 58 years, range 26-69 years). Younger patients receiving the symptom monitoring intervention experienced lower risk of ER visits [hazard ratio (HR) = 0.74, P = 0.011] and improved survival (HR = 0.76, P = 0.011) compared with younger patients receiving usual care. However, older patients did not experience significantly lower risk of ER visits (HR = 0.90, P = 0.613) or improved survival (HR = 1.06, P = 0.753) with the intervention. We found no moderation effects based on age for QoL and risk of hospitalizations.
Conclusions: Among patients with advanced cancer, age moderated the effects of an electronic symptom monitoring intervention on the risk of ER visits and survival, but not QoL. Symptom monitoring interventions may need to be tailored to the unique needs of older adults with cancer.
Keywords: advanced cancer; geriatric oncology; hospitalization; outcomes research; quality of life; symptoms.
Copyright © 2019 European Society for Medical Oncology. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
Comment in
-
Electronic symptom monitoring: not everyone fits the mold.Ann Oncol. 2020 Jan;31(1):13-14. doi: 10.1016/j.annonc.2019.10.016. Ann Oncol. 2020. PMID: 31912786 Free PMC article. No abstract available.
References
-
- Strasser F, Blum D, von Moos R et al. The effect of real-time electronic monitoring of patient-reported symptoms and clinical syndromes in outpatient workflow of medical oncologists: E-MOSAIC, a multicenter cluster-randomized phase III study (SAKK 95/06). Ann Oncol 2016; 27: 324–332. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical