Mps1-mediated release of Mad1 from nuclear pores ensures the fidelity of chromosome segregation
- PMID: 31913420
- PMCID: PMC7054998
- DOI: 10.1083/jcb.201906039
Mps1-mediated release of Mad1 from nuclear pores ensures the fidelity of chromosome segregation
Abstract
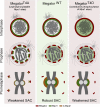
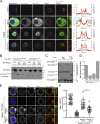
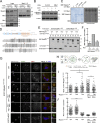
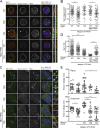
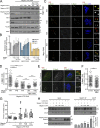
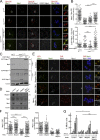
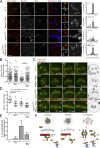
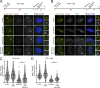
The spindle assembly checkpoint (SAC) relies on the recruitment of Mad1-C-Mad2 to unattached kinetochores but also on its binding to Megator/Tpr at nuclear pore complexes (NPCs) during interphase. However, the molecular underpinnings controlling the spatiotemporal redistribution of Mad1-C-Mad2 as cells progress into mitosis remain elusive. Here, we show that activation of Mps1 during prophase triggers Mad1 release from NPCs and that this is required for kinetochore localization of Mad1-C-Mad2 and robust SAC signaling. We find that Mps1 phosphorylates Megator/Tpr to reduce its interaction with Mad1 in vitro and in Drosophila cells. Importantly, preventing Mad1 from binding to Megator/Tpr restores Mad1 accumulation at kinetochores, the fidelity of chromosome segregation, and genome stability in larval neuroblasts of mps1-null mutants. Our findings demonstrate that the subcellular localization of Mad1 is tightly coordinated with cell cycle progression by kinetochore-extrinsic activity of Mps1. This ensures that both NPCs in interphase and kinetochores in mitosis can generate anaphase inhibitors to efficiently preserve genomic stability.
© 2020 Cunha-Silva et al.
Figures

References
-
- Bellen H.J., Levis R.W., He Y., Carlson J.W., Evans-Holm M., Bae E., Kim J., Metaxakis A., Savakis C., Schulze K.L., et al. . 2011. The Drosophila gene disruption project: progress using transposons with distinctive site specificities. Genetics. 188:731–743. 10.1534/genetics.111.126995 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

