Efficacy and Safety of Lumateperone for Treatment of Schizophrenia: A Randomized Clinical Trial
- PMID: 31913424
- PMCID: PMC6990963
- DOI: 10.1001/jamapsychiatry.2019.4379
Efficacy and Safety of Lumateperone for Treatment of Schizophrenia: A Randomized Clinical Trial
Erratum in
-
Data Errors in Results and Table 2.JAMA Psychiatry. 2020 Apr 1;77(4):438. doi: 10.1001/jamapsychiatry.2020.0055. JAMA Psychiatry. 2020. PMID: 32074262 Free PMC article. No abstract available.
Abstract
Importance: Individuals living with schizophrenia are affected by cardiometabolic, endocrine, and motor adverse effects of current antipsychotic medications. Lumateperone is a serotonin, dopamine, and glutamate modulator with the potential to treat schizophrenia with few adverse effects.
Objective: To examine the efficacy and safety of lumateperone for the short-term treatment of schizophrenia.
Design, setting, and participants: This randomized, double-blind, placebo-controlled, phase 3 clinical trial was conducted from November 13, 2014, to July 20, 2015, with data analyses performed from August 13 to September 15, 2015. Patients with schizophrenia who were aged 18 to 60 years and were experiencing an acute exacerbation of psychosis were enrolled from 12 clinical sites in the United States.
Interventions: Patients were randomized 1:1:1 (150 patients in each arm) to receive lumateperone tosylate, 60 mg; lumateperone tosylate, 40 mg (equivalent to 42 or 28 mg, respectively, of the active moiety lumateperone); or placebo once daily for 4 weeks.
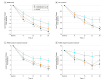
Main outcomes and measures: The prespecified primary efficacy end point was mean change from baseline to day 28 in the Positive and Negative Syndrome Scale (PANSS) total score vs placebo. The key secondary efficacy measure was the Clinical Global Impression-Severity of Illness (CGI-S) score. The PANSS subscale scores, social function, safety, and tolerability were also assessed.
Results: The study comprised 450 patients (mean [SD] age, 42.4 [10.2] years; 346 [77.1%] male; mean [SD] baseline PANSS score, 89.8 [10.3]; mean [SD] baseline CGI-S score, 4.8 [0.6]). In the prespecified modified intent-to-treat efficacy analysis (n = 435), 42 mg of lumateperone met the primary and key secondary efficacy objectives, demonstrating a statistically significant improvement vs placebo from baseline to day 28 on the PANSS total score (least-squares mean difference [LSMD], -4.2; 95% CI, -7.8 to -0.6; P = .02; effect size [ES], -0.3) and the CGI-S (LSMD, -0.3; 95% CI, -0.5 to -0.1; P = .003; ES, -0.4). For 28 mg of lumateperone, the LSMD from baseline to day 28 was -2.6 (95% CI, -6.2 to 1.1; P = .16; ES, -0.2) on the PANSS total score and -0.2 (95% CI, -0.5 to 0.0; P = .02; ES, -0.3) on the CGI-S. Both lumateperone doses were well tolerated without clinically significant treatment-emergent motor adverse effects or changes in cardiometabolic or endocrine factors vs placebo.
Conclusions and relevance: Lumateperone demonstrated efficacy for improving the symptoms of schizophrenia and had a favorable safety profile.
Trial registration: ClinicalTrials.gov identifier: NCT02282761.
Conflict of interest statement
Figures

Comment in
-
The Potential Role of Lumateperone-Something Borrowed? Something New?JAMA Psychiatry. 2020 Apr 1;77(4):343-344. doi: 10.1001/jamapsychiatry.2019.4265. JAMA Psychiatry. 2020. PMID: 31913409 No abstract available.
-
Effect Size and Blinding in Lumateperone Trial.JAMA Psychiatry. 2020 Jun 1;77(6):650-651. doi: 10.1001/jamapsychiatry.2020.0610. JAMA Psychiatry. 2020. PMID: 32320009 No abstract available.
-
Effect Size and Blinding in Lumateperone Trial-Reply.JAMA Psychiatry. 2020 Jun 1;77(6):651-652. doi: 10.1001/jamapsychiatry.2020.0613. JAMA Psychiatry. 2020. PMID: 32320025 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

