Eupatilin alleviates airway remodeling via regulating phenotype plasticity of airway smooth muscle cells
- PMID: 31913462
- PMCID: PMC6970064
- DOI: 10.1042/BSR20191445
Eupatilin alleviates airway remodeling via regulating phenotype plasticity of airway smooth muscle cells
Abstract
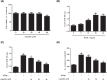
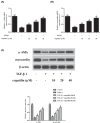
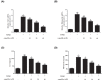
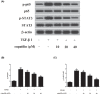
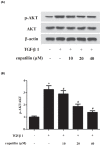
Childhood asthma is a common chronic airway disease, and its severe form remains a challenge. Eupatilin is a bioactive natural flavone that has been found to possess potential anti-asthma activity. However, the roles of eupatilin in asthma remain to be elucidated. In the present study, airway smooth muscle cells (ASMCs) were applied for the in vitro investigation since their phenotype plasticity make great contribution to airway remodeling during asthma pathogenesis. Our results showed that eupatilin suppressed the transforming growth factor β1 (TGF-β1)-induced proliferation and migration of ASMCs. Exposure of ASMCs to eupatilin increased the expressions of contractile markers smooth muscle α-actin (α-SMA) and myocardin, whereas expressions of extracellular matrix (ECM) proteins type I collagen (Coll I) and fibronectin were reduced. Furthermore, eupatilin treatment reversed the activation of nuclear factor-κ B (NF-κB), signal transducer and activator of transcription 3 (STAT3) and AKT pathways caused by TGF-β1 in ASMCs. These findings suggested that eupatilin might attenuate airway remodeling via regulating phenotype plasticity of ASMCs.
Keywords: Childhood asthma; airway remodeling; eupatilin; nuclear factor-kappa B (NF-κB); phenotype plasticity; signal transducer and activator of transcription 3 (STAT3).
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
Similar articles
-
Icariside II attenuates eosinophils-induced airway inflammation and remodeling via inactivation of NF-κB and STAT3 in an asthma mouse model.Exp Mol Pathol. 2020 Apr;113:104373. doi: 10.1016/j.yexmp.2020.104373. Epub 2020 Jan 7. Exp Mol Pathol. 2020. PMID: 31917285
-
TNFSF14, a novel target of miR-326, facilitates airway remodeling in airway smooth muscle cells via inducing extracellular matrix protein deposition and proliferation.Kaohsiung J Med Sci. 2020 Jul;36(7):508-514. doi: 10.1002/kjm2.12197. Epub 2020 Mar 2. Kaohsiung J Med Sci. 2020. PMID: 32118359 Free PMC article.
-
ABCA1 inhibits PDGF-induced proliferation and migration of rat airway smooth muscle cell through blocking TLR2/NF-κB/NFATc1 signaling.J Cell Biochem. 2018 Sep;119(9):7388-7396. doi: 10.1002/jcb.27046. Epub 2018 May 18. J Cell Biochem. 2018. PMID: 29775222
-
Rho/ROCK-MYOCD in regulating airway smooth muscle growth and remodeling.Am J Physiol Lung Cell Mol Physiol. 2021 Jul 1;321(1):L1-L5. doi: 10.1152/ajplung.00034.2021. Epub 2021 Apr 28. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 33909498 Review.
-
[Advances in pathogenesis of asthma airway remodeling and intervention mechanism of traditional Chinese medicine].Zhongguo Zhong Yao Za Zhi. 2025 Apr;50(8):2050-2070. doi: 10.19540/j.cnki.cjcmm.20241212.705. Zhongguo Zhong Yao Za Zhi. 2025. PMID: 40461218 Review. Chinese.
Cited by
-
Pretreatment with Eupatilin Attenuates Inflammation and Coagulation in Sepsis by Suppressing JAK2/STAT3 Signaling Pathway.J Inflamm Res. 2023 Mar 10;16:1027-1042. doi: 10.2147/JIR.S393850. eCollection 2023. J Inflamm Res. 2023. PMID: 36926276 Free PMC article.
-
Long noncoding RNA antisense noncoding RNA in the INK4 locus inhibition alleviates airway remodeling in asthma through the regulation of the microRNA-7-5p/early growth response factor 3 axis.Immun Inflamm Dis. 2023 Apr;11(4):e823. doi: 10.1002/iid3.823. Immun Inflamm Dis. 2023. PMID: 37102654 Free PMC article.
-
Eupatilin unveiled: An in-depth exploration of research advancements and clinical therapeutic prospects.J Transl Int Med. 2025 Apr 24;13(2):104-117. doi: 10.1515/jtim-2025-0016. eCollection 2025 Apr. J Transl Int Med. 2025. PMID: 40443403 Free PMC article.
-
Stevia Genus: Phytochemistry and Biological Activities Update.Molecules. 2021 May 6;26(9):2733. doi: 10.3390/molecules26092733. Molecules. 2021. PMID: 34066562 Free PMC article. Review.
-
Long non-coding RNA MEG3 knockdown represses airway smooth muscle cells proliferation and migration via sponging miR-143-3p/FGF9 in asthma.J Cardiothorac Surg. 2024 Jun 1;19(1):314. doi: 10.1186/s13019-024-02798-5. J Cardiothorac Surg. 2024. PMID: 38824534 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

