Putative human myometrial and fibroid stem-like cells have mesenchymal stem cell and endometrial stromal cell properties
- PMID: 31913469
- PMCID: PMC6993861
- DOI: 10.1093/humrep/dez247
Putative human myometrial and fibroid stem-like cells have mesenchymal stem cell and endometrial stromal cell properties
Abstract
Study question: Can endometrial stromal stem/progenitor cell markers, SUSD2 and CD146/CD140b, enrich for human myometrial and fibroid stem/progenitor cells?
Summary answer: SUSD2 enriches for myometrial and fibroid cells that have mesenchymal stem cell (MSC) characteristics and can also be induced to decidualise.
What is known already: Mesenchymal stem-like cells have been separately characterised in the endometrial stroma and myometrium and may contribute to diseases in their respective tissues.
Study design, size, duration: Normal myometrium, fibroids and endometrium were collected from hysterectomies with informed consent. Primary cells or tissues were used from at least three patient samples for each experiment.
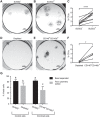
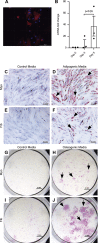
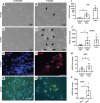
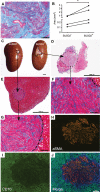
Participants/materials, setting, methods: Flow cytometry, immunohistochemistry and immunofluorescence were used to characterise tissues. In vitro colony formation in normoxic and hypoxic conditions, MSC lineage differentiation (osteogenic and adipogenic) and decidualisation were used to assess stem cell activity. Xenotransplantation into immunocompromised mice was used to determine in vivo stem-like activity. Endpoint measures included quantitative PCR, colony formation, trichrome, Oil Red O and alkaline phosphatase activity staining.
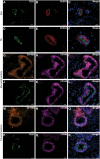
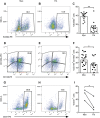
Main results and the role of chance: CD146+CD140b+ and/or SUSD2+ myometrial and fibroid cells were located in the perivascular region and formed more colonies in vitro compared to control cells and differentiated down adipogenic and osteogenic mesenchymal lineages in vitro. SUSD2+ myometrial cells had greater in vitro decidualisation potential, and SUSD2+ fibroid cells formed larger tumours in vivo compared to control cells.
Large-scale data: N/A.
Limitations, reasons for caution: Markers used in this study enrich for cells with stem/progenitor cell activity; however, they do not distinguish stem from progenitor cells. SUSD2+ myometrial cells express markers of decidualisation when treated in vitro, but in vivo assays are needed to fully demonstration their ability to decidualise.
Wider implications of the findings: These results suggest a possible common MSC for the endometrial stroma and myometrium, which could be the tumour-initiating cell for uterine fibroids.
Study funding/competing interest(s): These studies were supported by NIH grants to JMT (R01OD012206) and to ALP (F32HD081856). The authors certify that we have no conflicts of interest to disclose.
Keywords: endometrial stromal stem/progenitor cells; fibroids; mesenchymal stem cells; myometrium; stem/progenitor cells.
© The Author(s) 2020. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol 2005;288:276–283. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

