The olfactory G protein-coupled receptor (Olfr-78/OR51E2) modulates the intestinal response to colitis
- PMID: 31913697
- PMCID: PMC7099522
- DOI: 10.1152/ajpcell.00454.2019
The olfactory G protein-coupled receptor (Olfr-78/OR51E2) modulates the intestinal response to colitis
Abstract
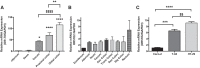
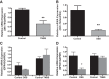
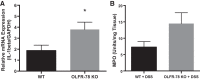
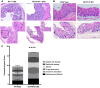
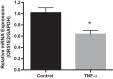
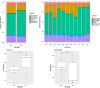
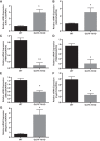
Olfactory receptor-78 (Olfr-78) is a recently identified G protein-coupled receptor activated by short-chain fatty acids acetate and propionate. A suggested role for this receptor exists in the prostate where it may influence chronic inflammatory response leading to intraepithelial neoplasia. Olfr-78 has also been shown to be expressed in mouse colon. Short-chain fatty acids and their receptors are well known to modulate inflammation in the gut. Considering this possibility, we first explored if colitis regulated Olfr-78 expression in the gut, where we observed a significant reduction in the expression of Olfr-78 transcript in mouse models of dextran sodium sulfate (DSS)- and 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis. To more directly test this, mice deficient in Olfr-78 were administered with DSS in water for 7 days and were found to have increased expression of IL-1β and inflammatory signs in colon compared with control mice. Next, we explored the expression of its human counterpart olfactory receptor family 51, subfamily E, member 2 (OR51E2) in human intestinal samples and observed that it was in fact also expressed in human colon samples. RNA sequence analysis revealed significant changes in the genes involved in infection, immunity, inflammation, and colorectal cancer between wild-type and Olfr-78 knockout mice. Collectively, our findings show that Olfr-78 is highly expressed in colon and downregulated in DSS- and TNBS-induced colitis, and DSS-treated Olfr-78 null mice had increased colonic expression of cytokine RNA levels, suggesting a potential role for this receptor in intestinal inflammation. Future investigations are needed to understand how Olfr-78/OR51E2 in both mouse and human intestine modulates gastrointestinal pathophysiology.
Keywords: Olfr-78; inflammation; intestine.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

