Mycobacterium tuberculosis Limits Host Glycolysis and IL-1β by Restriction of PFK-M via MicroRNA-21
- PMID: 31914380
- PMCID: PMC7764301
- DOI: 10.1016/j.celrep.2019.12.015
Mycobacterium tuberculosis Limits Host Glycolysis and IL-1β by Restriction of PFK-M via MicroRNA-21
Abstract
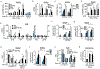
Increased glycolytic metabolism recently emerged as an essential process driving host defense against Mycobacterium tuberculosis (Mtb), but little is known about how this process is regulated during infection. Here, we observe repression of host glycolysis in Mtb-infected macrophages, which is dependent on sustained upregulation of anti-inflammatory microRNA-21 (miR-21) by proliferating mycobacteria. The dampening of glycolysis by miR-21 is mediated through targeting of phosphofructokinase muscle (PFK-M) isoform at the committed step of glycolysis, which facilitates bacterial growth by limiting pro-inflammatory mediators, chiefly interleukin-1β (IL-1β). Unlike other glycolytic genes, PFK-M expression and activity is repressed during Mtb infection through miR-21-mediated regulation, while other less-active isoenzymes dominate. Notably, interferon-γ (IFN-γ), which drives Mtb host defense, inhibits miR-21 expression, forcing an isoenzyme switch in the PFK complex, augmenting PFK-M expression and macrophage glycolysis. These findings place the targeting of PFK-M by miR-21 as a key node controlling macrophage immunometabolic function.
Keywords: glycolysis; interferon gamma; interleukin-1b; macrophage; metabolic reprogramming; miR-21; microRNA; mycobacterium tuberculosis; phosphofructokinase; tuberculosis.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures

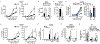

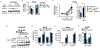
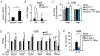

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

