Isolation and characterization of two O-methyltransferases involved in benzylisoquinoline alkaloid biosynthesis in sacred lotus (Nelumbo nucifera)
- PMID: 31914404
- PMCID: PMC7008365
- DOI: 10.1074/jbc.RA119.011547
Isolation and characterization of two O-methyltransferases involved in benzylisoquinoline alkaloid biosynthesis in sacred lotus (Nelumbo nucifera)
Abstract
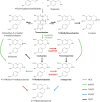
Benzylisoquinoline alkaloids (BIAs) are a major class of plant metabolites with many pharmacological benefits. Sacred lotus (Nelumbo nucifera) is an ancient aquatic plant of medicinal value because of antiviral and immunomodulatory activities linked to its constituent BIAs. Although more than 30 BIAs belonging to the 1-benzylisoquinoline, aporphine, and bisbenzylisoquinoline structural subclasses and displaying a predominant R-enantiomeric conformation have been isolated from N. nucifera, its BIA biosynthetic genes and enzymes remain unknown. Herein, we report the isolation and biochemical characterization of two O-methyltransferases (OMTs) involved in BIA biosynthesis in sacred lotus. Five homologous genes, designated NnOMT1-5 and encoding polypeptides sharing >40% amino acid sequence identity, were expressed in Escherichia coli Functional characterization of the purified recombinant proteins revealed that NnOMT1 is a regiospecific 1-benzylisoquinoline 6-O-methyltransferase (6OMT) accepting both R- and S-substrates, whereas NnOMT5 is mainly a 7-O-methyltransferase (7OMT), with relatively minor 6OMT activity and a strong stereospecific preference for S-enantiomers. Available aporphines were not accepted as substrates by either enzyme, suggesting that O-methylation precedes BIA formation from 1-benzylisoquinoline intermediates. Km values for NnOMT1 and NnOMT5 were 20 and 13 μm for (R,S)-norcoclaurine and (S)-N-methylcoclaurine, respectively, similar to those for OMTs from other BIA-producing plants. Organ-based correlations of alkaloid content, OMT activity in crude extracts, and OMT gene expression supported physiological roles for NnOMT1 and NnOMT5 in BIA metabolism, occurring primarily in young leaves and embryos of sacred lotus. In summary, our work identifies two OMTs involved in BIA metabolism in the medicinal plant N. nucifera.
Keywords: Nelumbo nucifera; O-methyltransferases; benzylisoquinoline alkaloids; enzyme catalysis; mass spectrometry (MS); natural product; natural product biosynthesis; plant biochemistry; plant molecular biology; regiospecificity; secondary metabolism; stereospecific.
© 2020 Menéndez-Perdomo and Facchini.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





Similar articles
-
Pathway elucidation and microbial synthesis of proaporphine and bis-benzylisoquinoline alkaloids from sacred lotus (Nelumbo nucifera).Metab Eng. 2023 May;77:162-173. doi: 10.1016/j.ymben.2023.03.010. Epub 2023 Mar 31. Metab Eng. 2023. PMID: 37004909
-
Benzylisoquinoline Alkaloids Biosynthesis in Sacred Lotus.Molecules. 2018 Nov 6;23(11):2899. doi: 10.3390/molecules23112899. Molecules. 2018. PMID: 30404216 Free PMC article. Review.
-
Elucidation of the (R)-enantiospecific benzylisoquinoline alkaloid biosynthetic pathways in sacred lotus (Nelumbo nucifera).Sci Rep. 2023 Feb 20;13(1):2955. doi: 10.1038/s41598-023-29415-0. Sci Rep. 2023. PMID: 36805479 Free PMC article.
-
Isolation and Characterization of O-methyltransferases Involved in the Biosynthesis of Glaucine in Glaucium flavum.Plant Physiol. 2015 Oct;169(2):1127-40. doi: 10.1104/pp.15.01240. Epub 2015 Aug 21. Plant Physiol. 2015. PMID: 26297140 Free PMC article.
-
Evolutionary and cellular webs in benzylisoquinoline alkaloid biosynthesis.Curr Opin Biotechnol. 2008 Apr;19(2):173-80. doi: 10.1016/j.copbio.2008.02.012. Epub 2008 Apr 7. Curr Opin Biotechnol. 2008. PMID: 18396034 Review.
Cited by
-
Catalytic promiscuity of O-methyltransferases from Corydalis yanhusuo leading to the structural diversity of benzylisoquinoline alkaloids.Hortic Res. 2022 Jul 6;9:uhac152. doi: 10.1093/hr/uhac152. eCollection 2022. Hortic Res. 2022. PMID: 36168544 Free PMC article.
-
O- and N-Methyltransferases in benzylisoquinoline alkaloid producing plants.Genes Genomics. 2024 Mar;46(3):367-378. doi: 10.1007/s13258-023-01477-4. Epub 2023 Dec 14. Genes Genomics. 2024. PMID: 38095842
-
Advances in the biosynthesis of naturally occurring benzylisoquinoline alkaloids.Front Plant Sci. 2025 Jan 30;16:1548471. doi: 10.3389/fpls.2025.1548471. eCollection 2025. Front Plant Sci. 2025. PMID: 39949415 Free PMC article. Review.
-
A Metabolomics Exploration of Young Lotus Seeds Using Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging.Molecules. 2025 Aug 1;30(15):3242. doi: 10.3390/molecules30153242. Molecules. 2025. PMID: 40807417 Free PMC article.
-
Mass Spectrometry Rearrangement Ions and Metabolic Pathway-Based Discovery of Indole Derivatives during the Aging Process in Citrus reticulata 'Chachi'.Foods. 2023 Dec 19;13(1):8. doi: 10.3390/foods13010008. Foods. 2023. PMID: 38201037 Free PMC article.
References
-
- Dastmalchi M., Park M. R., Morris J. S., and Facchini P. J. (2018) Family portraits: the enzymes behind benzylisoquinoline alkaloid diversity. Phytochem. Rev. 17, 249–277 10.1007/s11101-017-9519-z - DOI
-
- Singh A., Menéndez-Perdomo I. M., and Facchini P. J. (2019) Benzylisoquinoline alkaloid biosynthesis in opium poppy: and update. Phytochem. Rev. 18, 1457–1482 10.1007/s11101-019-09644-w - DOI
-
- Liscombe D. K., MacLeod B. P., Loukanina N., Nandi O. I., and Facchini P. J. (2005) Evidence for the monophyletic evolution of benzylisoquinoline alkaloid biosynthesis in angiosperms. Phytochemistry 66, 2501–2520 - PubMed
-
- Chen G., Zhu M., and Guo M. (2019) Research advances in traditional and modern use of Nelumbo nucifera: phytochemicals, health promoting activities and beyond. Crit. Rev. Food Sci. Nutr. 59, 189–209 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

