Crystal structures and biochemical analyses of the bacterial arginine dihydrolase ArgZ suggests a "bond rotation" catalytic mechanism
- PMID: 31914412
- PMCID: PMC7029115
- DOI: 10.1074/jbc.RA119.011752
Crystal structures and biochemical analyses of the bacterial arginine dihydrolase ArgZ suggests a "bond rotation" catalytic mechanism
Abstract
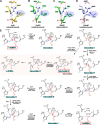
A recently discovered ornithine-ammonia cycle (OAC) serves as a conduit in the nitrogen storage and remobilization machinery in cyanobacteria. The OAC involves an arginine catabolic reaction catalyzed by the arginine dihydrolase ArgZ whose catalytic mechanism is unknown. Here we determined the crystal structures at 1.2-3.0 Å of unliganded ArgZ from the cyanobacterium Synechocystis sp. PCC6803 and of ArgZ complexed with its substrate arginine, a covalently linked reaction intermediate, or the reaction product ornithine. The structures reveal that a key residue, Asn71, in the ArgZ active center functions as the determinant distinguishing ArgZ from other members of the guanidino group-modifying enzyme superfamily. The structures, along with biochemical evidence from enzymatic assays coupled with electrospray ionization MS techniques, further suggest that ArgZ-catalyzed conversion of arginine to ornithine, ammonia, and carbon dioxide consists of two successive cycles of amine hydrolysis. Finally, we show that arginine dihydrolases are broadly distributed among bacteria and metazoans, suggesting that the OAC may be frequently used for redistribution of nitrogen from arginine catabolism or nitrogen fixation.
Keywords: arginine; arginine dihydrolase; arginine metabolism; cyanobacteria; enzyme catalysis; enzyme mechanism; enzyme structure; hydrolase; nitrogen metabolism; ornithine–ammonia cycle.
© 2020 Zhuang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




Comment in
-
Arginine catabolism enzyme AgrE/ArgZ likely involves a cyanobacterial specific factor.J Biol Chem. 2020 Mar 6;295(10):2915. doi: 10.1074/jbc.L120.012850. J Biol Chem. 2020. PMID: 32144146 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

