Preclinical evaluation of AT-527, a novel guanosine nucleotide prodrug with potent, pan-genotypic activity against hepatitis C virus
- PMID: 31914458
- PMCID: PMC6949113
- DOI: 10.1371/journal.pone.0227104
Preclinical evaluation of AT-527, a novel guanosine nucleotide prodrug with potent, pan-genotypic activity against hepatitis C virus
Abstract
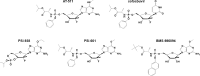
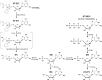
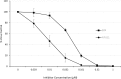
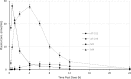
Despite the availability of highly effective direct-acting antiviral (DAA) regimens for the treatment of hepatitis C virus (HCV) infections, sustained viral response (SVR) rates remain suboptimal for difficult-to-treat patient populations such as those with HCV genotype 3, cirrhosis or prior treatment experience, warranting development of more potent HCV replication antivirals. AT-527 is the hemi-sulfate salt of AT-511, a novel phosphoramidate prodrug of 2'-fluoro-2'-C-methylguanosine-5'-monophosphate that has potent in vitro activity against HCV. The EC50 of AT-511, determined using HCV laboratory strains and clinical isolates with genotypes 1-5, ranged from 5-28 nM. The active 5'-triphosphate metabolite, AT-9010, specifically inhibited the HCV RNA-dependent RNA polymerase. AT-511 did not inhibit the replication of other selected RNA or DNA viruses in vitro. AT-511 was approximately 10-fold more active than sofosbuvir (SOF) against a panel of laboratory strains and clinical isolates of HCV genotypes 1-5 and remained fully active against S282T resistance-associated variants, with up to 58-fold more potency than SOF. In vitro, AT-511 did not inhibit human DNA polymerases or elicit cytotoxicity or mitochondrial toxicity at concentrations up to 100 μM. Unlike the other potent guanosine analogs PSI-938 and PSI-661, no mutagenic O6-alkylguanine bases were formed when incubated with cytochrome P450 (CYP) 3A4, and AT-511 had IC50 values ≥25 μM against a panel of CYP enzymes. In hepatocytes from multiple species, the active triphosphate was the predominant metabolite produced from the prodrug, with a half-life of 10 h in human hepatocytes. When given orally to rats and monkeys, AT-527 preferentially delivered high levels of AT-9010 in the liver in vivo. These favorable preclinical attributes support the ongoing clinical development of AT-527 and suggest that, when used in combination with an HCV DAA from a different class, AT-527 may increase SVR rates, especially for difficult-to-treat patient populations, and could potentially shorten treatment duration for all patients.
Conflict of interest statement
AM, XZ, KP and JS are employees of Atea Pharmaceuticals, Inc. and SG is a consultant for Atea Pharmaceuticals, Inc. AM and JS are co-inventors on the patent for AT-527. AT-527 is a product under development by Atea Pharmaceuticals, Inc. This does not alter our adherence to all PLOS ONE policies on sharing data and materials.
Figures



Similar articles
-
INX-08189, a phosphoramidate prodrug of 6-O-methyl-2'-C-methyl guanosine, is a potent inhibitor of hepatitis C virus replication with excellent pharmacokinetic and pharmacodynamic properties.Antimicrob Agents Chemother. 2011 May;55(5):1843-51. doi: 10.1128/AAC.01335-10. Epub 2011 Feb 28. Antimicrob Agents Chemother. 2011. PMID: 21357300 Free PMC article.
-
PSI-7851, a pronucleotide of beta-D-2'-deoxy-2'-fluoro-2'-C-methyluridine monophosphate, is a potent and pan-genotype inhibitor of hepatitis C virus replication.Antimicrob Agents Chemother. 2010 Aug;54(8):3187-96. doi: 10.1128/AAC.00399-10. Epub 2010 Jun 1. Antimicrob Agents Chemother. 2010. PMID: 20516278 Free PMC article.
-
Activity and the metabolic activation pathway of the potent and selective hepatitis C virus pronucleotide inhibitor PSI-353661.Antiviral Res. 2011 Aug;91(2):120-32. doi: 10.1016/j.antiviral.2011.05.003. Epub 2011 May 12. Antiviral Res. 2011. PMID: 21600932 Free PMC article.
-
Bemnifosbuvir (BEM, AT-527), a novel nucleotide analogue inhibitor of the hepatitis C virus NS5B polymerase.Expert Opin Investig Drugs. 2024 Jan;33(1):9-17. doi: 10.1080/13543784.2024.2305137. Epub 2024 Feb 12. Expert Opin Investig Drugs. 2024. PMID: 38265202 Review.
-
Nucleotide prodrugs for HCV therapy.Antivir Chem Chemother. 2011 Aug 23;22(1):23-49. doi: 10.3851/IMP1797. Antivir Chem Chemother. 2011. PMID: 21860070 Review.
Cited by
-
Human bronchopulmonary disposition and plasma pharmacokinetics of oral bemnifosbuvir (AT-527), an experimental guanosine nucleotide prodrug for COVID-19.J Antimicrob Chemother. 2024 Jun 3;79(6):1423-1431. doi: 10.1093/jac/dkae122. J Antimicrob Chemother. 2024. PMID: 38708557 Free PMC article. Clinical Trial.
-
Structure-Based Drug Design of RdRp Inhibitors against SARS-CoV-2.Top Curr Chem (Cham). 2023 Jun 15;381(5):22. doi: 10.1007/s41061-023-00432-x. Top Curr Chem (Cham). 2023. PMID: 37318607 Review.
-
SARS-CoV-2 RNA-dependent RNA polymerase as a therapeutic target for COVID-19.Expert Opin Ther Pat. 2021 Apr;31(4):325-337. doi: 10.1080/13543776.2021.1880568. Epub 2021 Mar 3. Expert Opin Ther Pat. 2021. PMID: 33475441 Free PMC article. Review.
-
AT-527, a Double Prodrug of a Guanosine Nucleotide Analog, Is a Potent Inhibitor of SARS-CoV-2 In Vitro and a Promising Oral Antiviral for Treatment of COVID-19.Antimicrob Agents Chemother. 2021 Mar 18;65(4):e02479-20. doi: 10.1128/AAC.02479-20. Print 2021 Mar 18. Antimicrob Agents Chemother. 2021. PMID: 33558299 Free PMC article.
-
Accelerated Preclinical Paths to Support Rapid Development of COVID-19 Therapeutics.Cell Host Microbe. 2020 Nov 11;28(5):638-645. doi: 10.1016/j.chom.2020.09.017. Epub 2020 Oct 1. Cell Host Microbe. 2020. PMID: 33152278 Free PMC article. Review.
References
-
- Hepatitis C Fact Sheet. World Health Organization 2019 [updated Jul 9, 2019. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c.]
-
- AASLD-IDSA. Recommendations for Testing, Managing, and Treating Hepatitis C 2019 [updated Nov 6, 2019. Available from: https://www.hcvguidelines.org/evaluate/testing-and-linkage.]
-
- Hosein S. HCV treatment in advanced liver disease CATIE2018 [Available from: https://www.catie.ca/en/treatmentupdate/treatmentupdate-227/hepatitis-c-....]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

