Partial Characterization of Two Cathepsin D Family Aspartic Peptidases of Clonorchis sinensis
- PMID: 31914521
- PMCID: PMC6960241
- DOI: 10.3347/kjp.2019.57.6.671
Partial Characterization of Two Cathepsin D Family Aspartic Peptidases of Clonorchis sinensis
Abstract
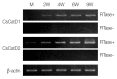
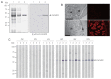
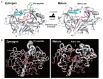
Cathepsin D (CatD, EC 3.4.23.5) is a member belonging to the subfamily of aspartic endopeptidases, which are classified into the MEROPS clan AA, family A1. Helminth parasites express a large set of different peptidases that play pivotal roles in parasite biology and pathophysiology. However, CatD is less well known than the other classes of peptidases in terms of biochemical properties and biological functions. In this study, we identified 2 novel CatDs (CsCatD1 and CsCatD2) of Clonorchis sinensis and partially characterized their properties. Both CsCatDs represent typical enzymes sharing amino acid residues and motifs that are tightly conserved in the CatD superfamily of proteins. Both CsCatDs showed similar patterns of expression in different developmental stages of C. sinensis, but CsCatD2 was also expressed in metacercariae. CsCatD2 was mainly expressed in the intestines and eggs of C. sinensis. Sera obtained from rats experimentally infected with C. sinensis reacted with recombinant CsCatD2 beginning 2 weeks after infection and the antibody titers were gradually increased by maturation of the parasite. Structural analysis of CsCatD2 revealed a bilobed enzyme structure consisting of 2 antiparallel β-sheet domains packed against each other forming a homodimeric structure. These results suggested a plausible biological role of CsCatD2 in the nutrition and reproduction of parasite and its potential utility as a serodiagnostic antigen in clonorchiasis.
Keywords: Clonorchis sinensis; aspartic peptidase; cathepsin D; egg; intestine; serodiagnostic antigen.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Characterization of a gut-associated asparaginyl endopeptidase of Clonorchis sinensis.Exp Parasitol. 2015 Jun;153:81-90. doi: 10.1016/j.exppara.2015.03.015. Epub 2015 Mar 24. Exp Parasitol. 2015. PMID: 25819296
-
A family of cathepsin F cysteine proteases of Clonorchis sinensis is the major secreted proteins that are expressed in the intestine of the parasite.Mol Biochem Parasitol. 2010 Mar;170(1):7-16. doi: 10.1016/j.molbiopara.2009.11.006. Epub 2009 Nov 22. Mol Biochem Parasitol. 2010. PMID: 19932715
-
Comparative biochemical and functional properties of two leucine aminopeptidases of Clonorchis sinensis.Mol Biochem Parasitol. 2012 Mar-Apr;182(1-2):17-26. doi: 10.1016/j.molbiopara.2011.11.009. Epub 2011 Nov 28. Mol Biochem Parasitol. 2012. PMID: 22155540
-
Identification of a serodiagnostic antigen, legumain, by immunoproteomic analysis of excretory-secretory products of Clonorchis sinensis adult worms.Proteomics. 2009 Jun;9(11):3066-78. doi: 10.1002/pmic.200700613. Proteomics. 2009. PMID: 19526557
-
Functional genes and proteins of Clonorchis sinensis.Korean J Parasitol. 2009 Oct;47 Suppl(Suppl):S59-68. doi: 10.3347/kjp.2009.47.S.S59. Korean J Parasitol. 2009. PMID: 19885336 Free PMC article. Review.
Cited by
-
Dopaminergic antagonists inhibit bile chemotaxis of adult Clonorchis sinensis and its egg production.PLoS Negl Trop Dis. 2020 Mar 30;14(3):e0008220. doi: 10.1371/journal.pntd.0008220. eCollection 2020 Mar. PLoS Negl Trop Dis. 2020. PMID: 32226018 Free PMC article.
-
Clonorchis sinensis excretory/secretory proteins ameliorate inflammation in rheumatoid arthritis and ankylosing spondylitis.Parasit Vectors. 2025 Mar 4;18(1):85. doi: 10.1186/s13071-025-06677-3. Parasit Vectors. 2025. PMID: 40038824 Free PMC article.
-
pH-Dependent Structural Dynamics of Cathepsin D-Family Aspartic Peptidase of Clonorchis sinensis.Pathogens. 2021 Sep 2;10(9):1128. doi: 10.3390/pathogens10091128. Pathogens. 2021. PMID: 34578162 Free PMC article.
-
An insight into the functional genomics and species classification of Eudiplozoon nipponicum (Monogenea, Diplozoidae), a haematophagous parasite of the common carp Cyprinus carpio.BMC Genomics. 2023 Jun 29;24(1):363. doi: 10.1186/s12864-023-09461-8. BMC Genomics. 2023. PMID: 37380941 Free PMC article.
-
Identification and Analysis of the Tegument Protein and Excretory-Secretory Products of the Carcinogenic Liver Fluke Clonorchis sinensis.Front Microbiol. 2020 Sep 23;11:555730. doi: 10.3389/fmicb.2020.555730. eCollection 2020. Front Microbiol. 2020. PMID: 33072014 Free PMC article.
References
-
- Lun ZR, Gasser RB, Lai DH, Li AX, Zhu XQ, Yu XB, Fang YY. Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis. 2005;5:31–41. - PubMed
-
- Vennervald BJ, Polman K. Helminths and malignancy. Parasite Immunol. 2009;31:686–696. - PubMed
-
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens-Part B: biological agents. Lancet Oncol. 2009;10:321–322. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

