Recovery of viable endocrine-specific cells and transcriptomes from human pancreatic islet-engrafted mice
- PMID: 31914605
- PMCID: PMC6972551
- DOI: 10.1096/fj.201901022RR
Recovery of viable endocrine-specific cells and transcriptomes from human pancreatic islet-engrafted mice
Abstract
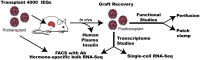
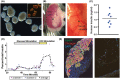
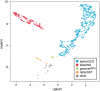
Human pancreatic islets engrafted into immunodeficient mice serve as an important model for in vivo human diabetes studies. Following engraftment, islet function can be monitored in vivo by measuring circulating glucose and human insulin; however, it will be important to recover viable cells for more complex graft analyses. Moreover, RNA analyses of dissected grafts have not distinguished which hormone-specific cell types contribute to gene expression. We developed a method for recovering live cells suitable for fluorescence-activated cell sorting from human islets engrafted in mice. Although yields of recovered islet cells were relatively low, the ratios of bulk-sorted β, α, and δ cells and their respective hormone-specific RNA-Seq transcriptomes are comparable pretransplant and posttransplant, suggesting that the cellular characteristics of islet grafts posttransplant closely mirror the original donor islets. Single-cell RNA-Seq transcriptome analysis confirms the presence of appropriate β, α, and δ cell subsets. In addition, ex vivo perifusion of recovered human islet grafts demonstrated glucose-stimulated insulin secretion. Viable cells suitable for patch-clamp analysis were recovered from transplanted human embryonic stem cell-derived β cells. Together, our functional and hormone-specific transcriptome analyses document the broad applicability of this system for longitudinal examination of human islet cells undergoing developmental/metabolic/pharmacogenetic manipulation in vivo and may facilitate the discovery of treatments for diabetes.
Keywords: L‐type voltage‐gated calcium channel; RNA‐Seq; graft recovery; insulin; β cell.
© 2019 The Authors. The FASEB Journal published by Wiley Periodicals, Inc. on behalf of Federation of American Societies for Experimental Biology.
Figures



Similar articles
-
In vitro characterization of neonatal, juvenile, and adult porcine islet oxygen demand, β-cell function, and transcriptomes.Xenotransplantation. 2018 Nov;25(6):e12432. doi: 10.1111/xen.12432. Epub 2018 Jul 27. Xenotransplantation. 2018. PMID: 30052287
-
Beta cells within single human islets originate from multiple progenitors.PLoS One. 2008;3(10):e3559. doi: 10.1371/journal.pone.0003559. Epub 2008 Oct 29. PLoS One. 2008. PMID: 18958289 Free PMC article.
-
Influence of VEGF on the viability of encapsulated pancreatic rat islets after transplantation in diabetic mice.Cell Transplant. 2003;12(6):627-35. doi: 10.3727/000000003108747109. Cell Transplant. 2003. PMID: 14579931
-
The Eye as a Transplantation Site to Monitor Pancreatic Islet Cell Plasticity.Front Endocrinol (Lausanne). 2021 Apr 23;12:652853. doi: 10.3389/fendo.2021.652853. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33967961 Free PMC article. Review.
-
Intraocular in vivo imaging of pancreatic islet cell physiology/pathology.Mol Metab. 2017 May 4;6(9):1002-1009. doi: 10.1016/j.molmet.2017.03.014. eCollection 2017 Sep. Mol Metab. 2017. PMID: 28951824 Free PMC article. Review.
Cited by
-
Retrieval and purification of human β cells from stem cell-derived islet engrafted mice.STAR Protoc. 2021 Jul 15;2(3):100675. doi: 10.1016/j.xpro.2021.100675. eCollection 2021 Sep 17. STAR Protoc. 2021. PMID: 34345868 Free PMC article.
-
Single-nucleus RNA sequencing of human pancreatic islets identifies novel gene sets and distinguishes β-cell subpopulations with dynamic transcriptome profiles.Genome Med. 2023 May 1;15(1):30. doi: 10.1186/s13073-023-01179-2. Genome Med. 2023. PMID: 37127706 Free PMC article.
-
Leptin modulates pancreatic β-cell membrane potential through Src kinase-mediated phosphorylation of NMDA receptors.J Biol Chem. 2020 Dec 11;295(50):17281-17297. doi: 10.1074/jbc.RA120.015489. Epub 2020 Oct 9. J Biol Chem. 2020. PMID: 33037073 Free PMC article.
-
Cell Heterogeneity and Paracrine Interactions in Human Islet Function: A Perspective Focused in β-Cell Regeneration Strategies.Front Endocrinol (Lausanne). 2021 Feb 3;11:619150. doi: 10.3389/fendo.2020.619150. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33613453 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

