Efficacy and safety of nintedanib in patients with advanced idiopathic pulmonary fibrosis
- PMID: 31914963
- PMCID: PMC6951000
- DOI: 10.1186/s12890-019-1030-4
Efficacy and safety of nintedanib in patients with advanced idiopathic pulmonary fibrosis
Abstract
Background: The two 52-week INPULSIS trials investigated nintedanib versus placebo in patients with IPF, FVC ≥50% predicted and DLco 30-79% predicted. The 24-week INSTAGE trial investigated nintedanib plus sildenafil versus nintedanib alone in patients with IPF and DLco ≤35% predicted. We used data from INPULSIS and INSTAGE to compare the effects of nintedanib in patients with IPF with less versus more severe impairment in gas exchange at baseline.
Methods: Analyses were conducted in patients treated with nintedanib alone in the INPULSIS and INSTAGE trials and in patients treated with placebo in the INPULSIS trials. Outcomes included the rate of decline in FVC over 24 weeks, the proportions of patients who had a confirmed or suspected idiopathic acute exacerbation over 24 weeks, deaths over 24 weeks, and adverse events. Analyses were descriptive.
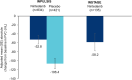
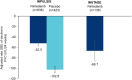
Results: In total, 638 and 136 patients received nintedanib alone in the INPULSIS and INSTAGE trials, respectively, and 423 patients received placebo in the INPULSIS trials. Rates of FVC decline were - 52.3 and - 66.7 mL/24 weeks in patients treated with nintedanib alone in INPULSIS and INSTAGE, respectively, and - 102.8 mL/24 weeks in patients treated with placebo in INPULSIS. Confirmed or suspected idiopathic acute exacerbations were reported in 0.6 and 3.7% of patients treated with nintedanib alone in INPULSIS and INSTAGE, respectively, and 2.1% of patients treated with placebo in INPULSIS. Deaths occurred in 2.0, 11.0 and 1.9% of patients in these groups, respectively. Diarrhoea adverse events were reported in 52.5 and 48.5% of patients treated with nintedanib alone in INPULSIS and INSTAGE, respectively, and 16.1% of patients treated with placebo in INPULSIS.
Conclusions: Based on data from the INSTAGE and INPULSIS trials, nintedanib had a similar effect on FVC decline over 24 weeks, and a similar safety and tolerability profile, in patients with IPF and more versus less severe impairment in gas exchange. These data support the use of nintedanib in patients with IPF who have advanced disease.
Trial registration: INPULSIS (NCT01335464 and NCT01335477); INSTAGE (NCT02802345).
Keywords: Clinical trial; Interstitial lung diseases; Tyrosine kinase inhibitor; Vital capacity.
Conflict of interest statement
LR reports grants and personal fees from Boehringer Ingelheim and Roche; and personal fees from Asahi Kasei, Biogen, Bristol-Myers Squibb, Celgene, CSL Behring, FibroGen, ImmuneWorks, Nitto, Pliant Therapeutics, Promedior, Respivant, and Toray. MK reports grants and personal fees from Boehringer Ingelheim, Gilead, GlaxoSmithKline, Prometic, and Roche; grants from Actelion, Alkermes, Pharmaxis, and RespiVert; and personal fees from Genoa, Indalo, and Third Pole. SJ has received fees, funding, or reimbursement for participation at meetings from Actelion, AIRB, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Gilead, GlaxoSmithKline, LVL, Mundipharma, Novartis, Pfizer, Roche, and Savara-Serendex. WAW reports grants to his institution from Boehringer Ingelheim and Roche and personal fees from Boehringer Ingelheim. BS, SS and MQ are employees of Boehringer Ingelheim, which funded the INPULSIS and INSTAGE trials; BS, SS and MQ were involved in the design of these trials, in the interpretation of the data and in writing the manuscript. BS was involved in analysing the data. GR has acted as a consultant for Bellerophon, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, FibroGen, Gilead, Nitto, Patara, Promedior, Sanofi, and Veracyte; has received a grant from the US National Institute of Health; and is a principal investigator and steering committee member for IPFnet studies.
Figures
References
-
- Kreuter M, Swigris J, Pittrow D, Geier S, Klotsche J, Prasse A, et al. The clinical course of idiopathic pulmonary fibrosis and its association to quality of life over time: longitudinal data from the INSIGHTS-IPF registry. Respir Res. 2019;20:59. doi: 10.1186/s12931-019-1020-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical