Exosomes overexpressing miR-34c inhibit malignant behavior and reverse the radioresistance of nasopharyngeal carcinoma
- PMID: 31915008
- PMCID: PMC6947927
- DOI: 10.1186/s12967-019-02203-z
Exosomes overexpressing miR-34c inhibit malignant behavior and reverse the radioresistance of nasopharyngeal carcinoma
Abstract
Background: Malignant behavior and radioresistance, which severely limits the efficacy of radiation therapy (RT) in nasopharyngeal carcinoma (NPC), are associated with tumor progression and poor prognosis. Mesenchymal stem cells (MSCs) are used as a therapeutic tool in a variety of tumors. The aim of this study was to reveal the effect of tumor suppressor microRNA-34c-5p (miR-34c) on NPC development and radioresistance, as well as to confirm that exosomes derived from MSCs overexpressing miR-34c restore the sensitivity to radiotherapy in NPCs.
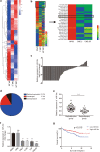
Methods: Potentially active microRNAs were screened by cell sequencing, Gene Expression Omnibus (GEO) database analysis, and analysis of clinical serum samples from 70 patients. The expression of genes and proteins was detected by Western blotting, quantitative reverse transcription PCR (qRT-PCR), and immunohistochemistry (IHC). Proliferation, apoptosis, invasion, migration and radioresistance of NPC were detected. Luciferase reporter assays were used to verify the interactions of microRNAs with their downstream targets. MSCs exosomes were isolated by ultrafiltration and verified by electron microscopy and nanoparticle tracking technology.
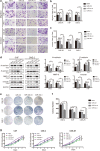
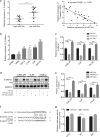
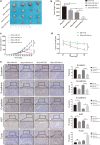
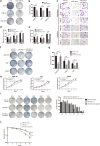
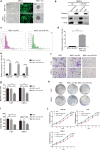
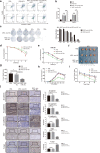
Results: The expression of miR-34c was associated with the occurrence and radiation resistance of NPC. In vitro and in vivo experiments indicated that overexpression of miR-34c inhibit malignant behavior such as invasion, migration, proliferation and epithelial-mesenchymal transition (EMT) in NPCs by targeting β-Catenin. In addition, we found alleviated radioresistance upon miR-34c overexpression or β-catenin knockdown in NPCs. Exosomes derived from miR-34c-transfected MSCs attenuated NPC invasion, migration, proliferation and EMT. Moreover, miR-34c-overexpressing exosomes drastically increased radiation-induced apoptosis in NPC cells.
Conclusion: miR-34c is a tumor suppressor miR in NPC, which inhibits malignant behavior as well as radioresistance of tumor. Therefore, exogenous delivery of miR-34c to NPCs via MSC exosomes inhibits tumor progression and increases the efficiency of RT. Combination IR with miR-34c-overexpressing exosomes may be effective treatment for radioresistant NPCs.
Keywords: EMT; Nasopharyngeal carcinoma; Radioresistance; microRNA-34c; β-Catenin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
MiR-34c downregulation leads to SOX4 overexpression and cisplatin resistance in nasopharyngeal carcinoma.BMC Cancer. 2020 Jun 26;20(1):597. doi: 10.1186/s12885-020-07081-z. BMC Cancer. 2020. PMID: 32586280 Free PMC article.
-
Up-regulation of miR-34c-5p inhibits nasopharyngeal carcinoma cells by mediating NOTCH1.Biosci Rep. 2020 Jun 26;40(6):BSR20200302. doi: 10.1042/BSR20200302. Biosci Rep. 2020. Retraction in: Biosci Rep. 2021 Oct 29;41(10):BSR-2020-0302_RET. doi: 10.1042/BSR-2020-0302_RET. PMID: 32458967 Free PMC article. Retracted.
-
MicroRNA-296-5p inhibits cell metastasis and invasion in nasopharyngeal carcinoma by reversing transforming growth factor-β-induced epithelial-mesenchymal transition.Cell Mol Biol Lett. 2020 Nov 3;25(1):49. doi: 10.1186/s11658-020-00240-x. Cell Mol Biol Lett. 2020. PMID: 33292168 Free PMC article.
-
The Role of Cyclin d1 in Radiotherapy Resistance of Advance Stage Nasopharyngeal Carcinoma: A Systematic Review.Asian Pac J Cancer Prev. 2024 Jul 1;25(7):2211-2218. doi: 10.31557/APJCP.2024.25.7.2211. Asian Pac J Cancer Prev. 2024. PMID: 39068551 Free PMC article.
-
Exosomes in nasopharyngeal carcinoma.Clin Chim Acta. 2021 Dec;523:355-364. doi: 10.1016/j.cca.2021.10.013. Epub 2021 Oct 16. Clin Chim Acta. 2021. PMID: 34666030 Review.
Cited by
-
MicroRNA-613 Enhances Nasopharyngeal Carcinoma Cell Radiosensitivity via the DNA Methyltransferase 3B/Tissue Inhibitor of Matrix Metalloproteinase-3/Signal Transducer and Activator of Transcription-1/Forkhead Box O-1 Axis.Dis Markers. 2022 Aug 26;2022:5699275. doi: 10.1155/2022/5699275. eCollection 2022. Dis Markers. 2022. PMID: 36061358 Free PMC article.
-
LncRNA CASC19 Enhances the Radioresistance of Nasopharyngeal Carcinoma by Regulating the miR-340-3p/FKBP5 Axis.Int J Mol Sci. 2023 Feb 3;24(3):3047. doi: 10.3390/ijms24033047. Int J Mol Sci. 2023. PMID: 36769373 Free PMC article.
-
Extracellular Vesicles in the Progression and Therapeutic Resistance of Nasopharyngeal Carcinoma.Cancers (Basel). 2022 May 4;14(9):2289. doi: 10.3390/cancers14092289. Cancers (Basel). 2022. PMID: 35565418 Free PMC article. Review.
-
Application of small extracellular vesicles in the diagnosis and prognosis of nasopharyngeal carcinoma.Front Cell Dev Biol. 2023 Mar 10;11:1100941. doi: 10.3389/fcell.2023.1100941. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36968209 Free PMC article. Review.
-
Harnessing genetically engineered cell membrane-derived vesicles as biotherapeutics.Extracell Vesicles Circ Nucl Acids. 2024 Jan 31;5(1):44-63. doi: 10.20517/evcna.2023.58. eCollection 2024. Extracell Vesicles Circ Nucl Acids. 2024. PMID: 39698409 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

