SOD2 ameliorates pulmonary hypertension in a murine model of sleep apnea via suppressing expression of NLRP3 in CD11b+ cells
- PMID: 31915037
- PMCID: PMC6951024
- DOI: 10.1186/s12931-019-1270-0
SOD2 ameliorates pulmonary hypertension in a murine model of sleep apnea via suppressing expression of NLRP3 in CD11b+ cells
Abstract
Background: High prevalence of obstructive sleep apnea (OSA) in the pulmonary hypertension (PH) population suggests that chronic intermittent hypoxia (CIH) is an important pathogenic factor of PH. However, the exact mechanism of CIH induced PH is not clear. One of the molecules that plays a key role in regulating pulmonary artery function under hypoxic conditions is superoxide dismutase 2 (SOD2).
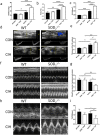
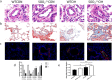
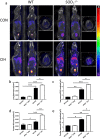
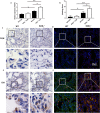
Methods: Our study utilized heterozygous SOD2-/+ mice firstly in CIH model to explore the exact role of SOD2 in CIH causing PH. Expression of SOD2 was analyzed in CIH model. Echocardiography and pulmonary hypertension were measured in wild type (WT) and SOD2-/+ mice under normal air or CIH condition. Hematoxylin-Eosin (H&E) staining and masson staining were carried out to evaluate pulmonary vascular muscularization and remodeling. Micro-PET scanning of in vivo 99mTc-labelled- MAG3-anti-CD11b was applied to assess CD11b in quantification and localization. Level of nod-like receptor pyrin domain containing 3 (NLRP3) was analyzed by real time PCR and immunohistochemistry (IHC).
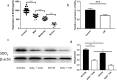
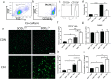
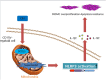
Results: Results showed that SOD2 was down-regulated in OSA/CIH model. Deficiency of SOD2 aggravated CIH induced pulmonary hypertension and pulmonary vascular hypertrophy. CD11b+ cells, especially monocytic myeloid cell line-Ly6C+Ly6G- cells, were increased in the lung, bone marrow and the blood under CIH condition, and down-regulated SOD2 activated NLRP3 in CD11b+ cells. SOD2-deficient-CD11b+ myeloid cells promoted the apoptosis resistance and over-proliferation of human pulmonary artery smooth muscle cells (PASMCs) via up-regulating NLRP3.
Conclusion: CIH induced down-regulating of SOD2 increased pulmonary hypertension and vascular muscularization. It could be one of the mechanism of CIH leading to PH.
Keywords: CD11b; Chronic intermittent hypoxia; NLRP3; Pulmonary hypertension; SOD2.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
STAT6 deficiency mitigates the severity of pulmonary arterial hypertension caused by chronic intermittent hypoxia by suppressing Th2-inducing cytokines.Respir Res. 2025 Jan 13;26(1):13. doi: 10.1186/s12931-024-03062-z. Respir Res. 2025. PMID: 39806384 Free PMC article.
-
Heterozygous SOD2 deletion deteriorated chronic intermittent hypoxia-induced lung inflammation and vascular remodeling through mtROS-NLRP3 signaling pathway.Acta Pharmacol Sin. 2020 Sep;41(9):1197-1207. doi: 10.1038/s41401-019-0349-y. Epub 2020 Feb 17. Acta Pharmacol Sin. 2020. PMID: 32066884 Free PMC article.
-
NLRP3 Deficiency Protects Against Intermittent Hypoxia-Induced Neuroinflammation and Mitochondrial ROS by Promoting the PINK1-Parkin Pathway of Mitophagy in a Murine Model of Sleep Apnea.Front Immunol. 2021 Feb 24;12:628168. doi: 10.3389/fimmu.2021.628168. eCollection 2021. Front Immunol. 2021. PMID: 33717152 Free PMC article.
-
Potential Contribution of Carotid Body-Induced Sympathetic and Renin-Angiotensin System Overflow to Pulmonary Hypertension in Intermittent Hypoxia.Curr Hypertens Rep. 2019 Oct 10;21(11):89. doi: 10.1007/s11906-019-0995-y. Curr Hypertens Rep. 2019. PMID: 31599367 Review.
-
Neural regulation of hypoxia-inducible factors and redox state drives the pathogenesis of hypertension in a rodent model of sleep apnea.J Appl Physiol (1985). 2015 Nov 15;119(10):1152-6. doi: 10.1152/japplphysiol.00162.2015. Epub 2015 May 7. J Appl Physiol (1985). 2015. PMID: 25953833 Free PMC article. Review.
Cited by
-
STAT6 deficiency mitigates the severity of pulmonary arterial hypertension caused by chronic intermittent hypoxia by suppressing Th2-inducing cytokines.Respir Res. 2025 Jan 13;26(1):13. doi: 10.1186/s12931-024-03062-z. Respir Res. 2025. PMID: 39806384 Free PMC article.
-
Signaling pathways and targeted therapy for pulmonary hypertension.Signal Transduct Target Ther. 2025 Jul 1;10(1):207. doi: 10.1038/s41392-025-02287-8. Signal Transduct Target Ther. 2025. PMID: 40588471 Free PMC article. Review.
-
Metabolomics of Artichoke Bud Extract in Spontaneously Hypertensive Rats.ACS Omega. 2021 Jul 12;6(29):18610-18622. doi: 10.1021/acsomega.1c01135. eCollection 2021 Jul 27. ACS Omega. 2021. PMID: 34337201 Free PMC article.
-
Oxidative stress and reactive oxygen species in otorhinolaryngological diseases: insights from pathophysiology to targeted antioxidant therapies.Redox Rep. 2025 Dec;30(1):2458942. doi: 10.1080/13510002.2025.2458942. Epub 2025 Feb 2. Redox Rep. 2025. PMID: 39894944 Free PMC article. Review.
-
Disulfiram attenuates hypoxia-induced pulmonary hypertension by inhibiting GSDMD cleavage and pyroptosis in HPASMCs.Respir Res. 2022 Dec 16;23(1):353. doi: 10.1186/s12931-022-02279-0. Respir Res. 2022. PMID: 36527086 Free PMC article.
References
-
- Pham Luu V., Miele Catherine H., Schwartz Noah G., Arias Rafael S., Rattner Adi, Gilman Robert H., Miranda J. Jaime, Polotsky Vsevolod Y., Checkley William, Schwartz Alan R. Cardiometabolic correlates of sleep disordered breathing in Andean highlanders. European Respiratory Journal. 2017;49(6):1601705. doi: 10.1183/13993003.01705-2016. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

