Leishmania braziliensis prostaglandin F2α synthase impacts host infection
- PMID: 31915065
- PMCID: PMC6950890
- DOI: 10.1186/s13071-020-3883-z
Leishmania braziliensis prostaglandin F2α synthase impacts host infection
Abstract
Background: Prostaglandins (PG) are lipid mediators derived from arachidonic acid metabolism. They are involved in cellular processes such as inflammation and tissue homeostasis. PG production is not restricted to multicellular organisms. Trypanosomatids also synthesize several metabolites of arachidonic acid. Nevertheless, their biological role in these early-branching parasites and their role in host-parasite interaction are not well elucidated. Prostaglandin F2α synthase (PGF2S) has been observed in the Leishmania braziliensis secreted proteome and in L. donovani extracellular vesicles. Furthermore, we previously reported a positive correlation between L. braziliensis PGF2S (LbrPGF2S) expression and pathogenicity in mice.
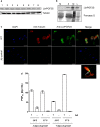
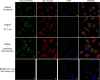
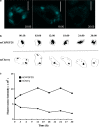
Methods: LbrPGF2S gene expression and PGF2α synthesis in promastigotes were detected and quantified by western blotting and EIA assay kit, respectively. To investigate LbrPGF2S localization in amastigotes during bone marrow-derived macrophage infection, parasites expressing mCherry-LbrPGF2S were generated and followed by time-lapse imaging for 48 h post-infection. PGF2S homolog sequences from Leishmania and humans were analyzed in silico using ClustalW on Geneious v6 and EMBOSS Needle.
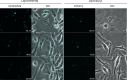
Results: Leishmania braziliensis promastigotes synthesize prostaglandin F2α in the presence of arachidonic acid, with peak production in the stationary growth phase under heat stress. LbrPGF2S is a cytoplasmic protein enriched in the secretory site of the parasite cell body, the flagellar pocket. It is an enzyme constitutively expressed throughout promastigote development, but overexpression of LbrPGF2S leads to an increase of infectivity in vitro. The data suggest that LbrPGF2S may be released from intracellular amastigotes into the cytoplasm of bone marrow-derived macrophages over a 48-hour infection period, using time-lapse microscopy and mCherry-PGF2S (mChPGF2S)-expressing parasites.
Conclusions: LbrPGF2S, a parasite-derived protein, is targeted to the host cell cytoplasm. The putative transfer of this enzyme, involved in pro-inflammatory lipid mediator synthesis, to the host cell suggests a potential role in host-parasite interaction and may partially explain the increased pathogenicity associated with overexpression of LbrPGF2S in L. braziliensis. Our data provide valuable insights to help understand the importance of parasite-derived lipid mediators in pathogenesis.
Keywords: Host-parasite; LbrPGF2S; Leishmania braziliensis; Macrophage; PGF2α.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Alves-Ferreira EV, Toledo JS, De Oliveira AH, Ferreira TR, Ruy PC, Pinzan CF, et al. Differential gene expression and infection profiles of cutaneous and mucosal Leishmania braziliensis isolates from the same patient. PLoS Negl Trop Dis. 2015;9:e0004018. doi: 10.1371/journal.pntd.0004018. - DOI - PMC - PubMed
-
- Moen SO, Fairman JW, Barnes SR, Sullivan A, Nakazawa-Hewitt S, Van Voorhis WC, et al. Structures of prostaglandin F synthase from the protozoa Leishmania major and Trypanosoma cruzi with NADP. Acta Crystallogr F Struct Biol Commun. 2015;71:609–614. doi: 10.1107/S2053230X15006883. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 2011/02040-4/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2013/50219-9/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 305775/2013-8/Conselho Nacional de Desenvolvimento Científico e Tecnológico
- MR/L00092X/1/MRC_/Medical Research Council/United Kingdom
- MR/N017633/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources

