Tumor Cell-Derived IL1β Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer
- PMID: 31915130
- PMCID: PMC7302116
- DOI: 10.1158/0008-5472.CAN-19-2080
Tumor Cell-Derived IL1β Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer
Abstract
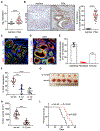
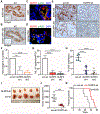
Pancreatic ductal adenocarcinoma (PDA) is an aggressive malignancy typified by a highly stromal and weakly immunogenic tumor microenvironment that promotes tumor evolution and contributes to therapeutic resistance. Here, we demonstrate that PDA tumor cell-derived proinflammatory cytokine IL1β is essential for the establishment of the protumorigenic PDA microenvironment. Tumor cell-derived IL1β promoted the activation and secretory phenotype of quiescent pancreatic stellate cells and established an immunosuppressive milieu mediated by M2 macrophages, myeloid-derived suppressor cells, CD1dhiCD5+ regulatory B cells, and Th17 cells. Loss of tumor cell-derived IL1 signaling in tumor stroma enabled intratumoral infiltration and activation of CD8+ cytotoxic T cells, attenuated growth of pancreatic neoplasia, and conferred survival advantage to PDA-bearing mice. Accordingly, antibody-mediated neutralization of IL1β significantly enhanced the antitumor activity of α-PD-1 and was accompanied by increased tumor infiltration of CD8+ T cells. Tumor cell expression of IL1β in vivo was driven by microbial-dependent activation of toll-like receptor 4 (TLR4) signaling and subsequent engagement of the NLRP3 inflammasome. Collectively, these findings identify a hitherto unappreciated role for tumor cell-derived IL1β in orchestrating an immune-modulatory program that supports pancreatic tumorigenesis. SIGNIFICANCE: These findings identify a new modality for immune evasion in PDA that depends on IL1β production by tumor cells through TLR4-NLRP3 inflammasome activation. Targeting this axis might provide an effective PDA therapeutic strategy.
©2020 American Association for Cancer Research.
Conflict of interest statement
Figures




References
-
- Simoes PK, Olson SH, Saldia A, Kurtz RC. Epidemiology of pancreatic adenocarcinoma. Chin Clin Oncol 2017;6:24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

