Effect of early palliative care for patients with glioblastoma (EPCOG): a randomised phase III clinical trial protocol
- PMID: 31915175
- PMCID: PMC6955518
- DOI: 10.1136/bmjopen-2019-034378
Effect of early palliative care for patients with glioblastoma (EPCOG): a randomised phase III clinical trial protocol
Abstract
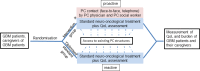
INTRODUCTION: Randomised controlled trials (RCTs) have shown a positive effect of early integration of palliative care (EIPC) in various advanced cancer entities regarding patients' quality of life (QoL), survival, mood, caregiver burden and reduction of aggressiveness of treatment near the end of life. However, RCTs investigating the positive effect of EIPC for patients suffering from glioblastoma multiforme (GBM) are lacking. After modelling work identifying the specific needs of GBM patients and their caregivers, the aim of this study is to investigate the impact of EIPC in this particular patient group. METHODS AND ANALYSIS: The recruitment period of this multicenter RCT started in May 2019. GBM patients (n=214) and their caregivers will be randomly assigned to either the intervention group (receiving proactive EIPC on a monthly basis) or the control group (receiving treatment according to international standards and additional, regular assessment of QoL ('optimised' standard care)).The primary outcome is QoL assessed by subscales of the Functional Assessment of Cancer Therapy for brain tumour (FACT-Br) from baseline to 6 months of treatment. Secondary outcomes are changes in QoL after 12 (end of intervention), 18 and 24 months (end of follow-up), the full FACT-Br scale, patients' palliative care needs, depression/anxiety, cognitive impairment, caregiver burden, healthcare use, cost-effectiveness and overall survival. ETHICS AND DISSEMINATION: The study will be conducted in accordance with the Declaration of Helsinki and has been approved by the local ethics committees of the University Clinics of Cologne, Aachen, Bonn, Freiburg and Munich (LMU). Results of the trial will be submitted for publication in a peer-reviewed, open access journal and disseminated through presentations at conferences. TRIAL REGISTRATION NUMBER: German Register for Clinical Studies (DRKS) (DRKS00016066); Pre-results.
Keywords: RCT; caregiver burden; early integration of palliative care; glioblastoma; palliative care; quality of life.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
