First characterization of glucose flux through the hexosamine biosynthesis pathway (HBP) in ex vivo mouse heart
- PMID: 31915250
- PMCID: PMC7029105
- DOI: 10.1074/jbc.RA119.010565
First characterization of glucose flux through the hexosamine biosynthesis pathway (HBP) in ex vivo mouse heart
Abstract
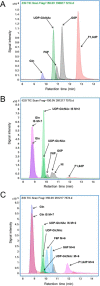
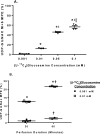
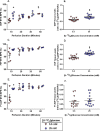
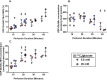
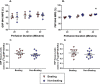
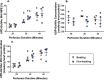
The hexosamine biosynthesis pathway (HBP) branches from glycolysis and forms UDP-GlcNAc, the moiety for O-linked β-GlcNAc (O-GlcNAc) post-translational modifications. An inability to directly measure HBP flux has hindered our understanding of the factors regulating protein O-GlcNAcylation. Our goals in this study were to (i) validate a LC-MS method that assesses HBP flux as UDP-GlcNAc (13C)-molar percent enrichment (MPE) and concentration and (ii) determine whether glucose availability or workload regulate cardiac HBP flux. For (i), we perfused isolated murine working hearts with [U-13C6]glucosamine (1, 10, 50, or 100 μm), which bypasses the rate-limiting HBP enzyme. We observed a concentration-dependent increase in UDP-GlcNAc levels and MPE, with the latter reaching a plateau of 56.3 ± 2.9%. For (ii), we perfused isolated working hearts with [U-13C6]glucose (5.5 or 25 mm). Glycolytic efflux doubled with 25 mm [U-13C6]glucose; however, the calculated HBP flux was similar among the glucose concentrations at ∼2.5 nmol/g of heart protein/min, representing ∼0.003-0.006% of glycolysis. Reducing cardiac workload in beating and nonbeating Langendorff perfusions had no effect on the calculated HBP flux at ∼2.3 and 2.5 nmol/g of heart protein/min, respectively. To the best of our knowledge, this is the first direct measurement of glucose flux through the HBP in any organ. We anticipate that these methods will enable foundational analyses of the regulation of HBP flux and protein O-GlcNAcylation. Our results suggest that in the healthy ex vivo perfused heart, HBP flux does not respond to acute changes in glucose availability or cardiac workload.
Keywords: O-linked N-acetylglucosamine (O-GlcNAc); UDP-GlcNAc; carbohydrate metabolism; cardiac metabolism; glucosamine; glucose; glucose metabolism; hexosamine biosynthesis pathway; metabolic flux; post-translational modification (PTM); protein glycosylation.
© 2020 Olson et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





Similar articles
-
Epithelial Mesenchymal Transition Induces Aberrant Glycosylation through Hexosamine Biosynthetic Pathway Activation.J Biol Chem. 2016 Jun 17;291(25):12917-29. doi: 10.1074/jbc.M116.729236. Epub 2016 Apr 18. J Biol Chem. 2016. PMID: 27129262 Free PMC article.
-
Hexosamine biosynthetic pathway promotes the antiviral activity of SAMHD1 by enhancing O-GlcNAc transferase-mediated protein O-GlcNAcylation.Theranostics. 2021 Jan 1;11(2):805-823. doi: 10.7150/thno.50230. eCollection 2021. Theranostics. 2021. PMID: 33391506 Free PMC article.
-
Acute regulation of cardiac metabolism by the hexosamine biosynthesis pathway and protein O-GlcNAcylation.PLoS One. 2011 Apr 11;6(4):e18417. doi: 10.1371/journal.pone.0018417. PLoS One. 2011. PMID: 21494549 Free PMC article.
-
Hexosamines, insulin resistance, and the complications of diabetes: current status.Am J Physiol Endocrinol Metab. 2006 Jan;290(1):E1-E8. doi: 10.1152/ajpendo.00329.2005. Am J Physiol Endocrinol Metab. 2006. PMID: 16339923 Free PMC article. Review.
-
The Hexosamine Biosynthesis Pathway: Regulation and Function.Genes (Basel). 2023 Apr 18;14(4):933. doi: 10.3390/genes14040933. Genes (Basel). 2023. PMID: 37107691 Free PMC article. Review.
Cited by
-
Integration of O-GlcNAc into Stress Response Pathways.Cells. 2022 Nov 5;11(21):3509. doi: 10.3390/cells11213509. Cells. 2022. PMID: 36359905 Free PMC article. Review.
-
O-GlcNAcylation: cellular physiology and therapeutic target for human diseases.MedComm (2020). 2023 Dec 19;4(6):e456. doi: 10.1002/mco2.456. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 38116061 Free PMC article. Review.
-
GFAT2 mediates cardiac hypertrophy through HBP-O-GlcNAcylation-Akt pathway.iScience. 2021 Nov 26;24(12):103517. doi: 10.1016/j.isci.2021.103517. eCollection 2021 Dec 17. iScience. 2021. PMID: 34934932 Free PMC article.
-
Altered O-GlcNAcylation and mitochondrial dysfunction, a molecular link between brain glucose dysregulation and sporadic Alzheimer's disease.Neural Regen Res. 2023 Apr;18(4):779-783. doi: 10.4103/1673-5374.354515. Neural Regen Res. 2023. PMID: 36204837 Free PMC article.
-
O-GlcNAcylation of TRIM29 and OGT translation forms a feedback loop to promote adaptive response of PDAC cells to glucose deficiency.Cell Oncol (Dordr). 2024 Jun;47(3):1025-1041. doi: 10.1007/s13402-023-00915-5. Epub 2024 Feb 12. Cell Oncol (Dordr). 2024. PMID: 38345749
References
-
- Torres C. R., and Hart G. W. (1984) Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes: evidence for O-linked GlcNAc. J. Biol. Chem. 259, 3308–3317 - PubMed
-
- Zhu W. Z., El-Nachef D., Yang X., Ledee D., and Olson A. K. (2019) O-GlcNAc transferase promotes compensated cardiac function and protein kinase A O-GlcNAcylation during early and established pathological hypertrophy from pressure overload. J. Am. Heart Assoc. 8, e011260 10.1161/JAHA.118.011260 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

