A Genome-Wide CRISPR-Cas9 Screen Identifies the Dolichol-Phosphate Mannose Synthase Complex as a Host Dependency Factor for Dengue Virus Infection
- PMID: 31915280
- PMCID: PMC7081898
- DOI: 10.1128/JVI.01751-19
A Genome-Wide CRISPR-Cas9 Screen Identifies the Dolichol-Phosphate Mannose Synthase Complex as a Host Dependency Factor for Dengue Virus Infection
Abstract
Dengue virus (DENV) is a mosquito-borne flavivirus responsible for dengue disease, a major human health concern for which no specific therapies are available. Like other viruses, DENV relies heavily on the host cellular machinery for productive infection. In this study, we performed a genome-wide CRISPR-Cas9 screen using haploid HAP1 cells to identify host genes important for DENV infection. We identified DPM1 and -3, two subunits of the endoplasmic reticulum (ER) resident dolichol-phosphate mannose synthase (DPMS) complex, as host dependency factors for DENV and other related flaviviruses, such as Zika virus (ZIKV). The DPMS complex catalyzes the synthesis of dolichol-phosphate mannose (DPM), which serves as mannosyl donor in pathways leading to N-glycosylation, glycosylphosphatidylinositol (GPI) anchor biosynthesis, and C- or O-mannosylation of proteins in the ER lumen. Mutation in the DXD motif of DPM1, which is essential for its catalytic activity, abolished DPMS-mediated DENV infection. Similarly, genetic ablation of ALG3, a mannosyltransferase that transfers mannose to lipid-linked oligosaccharide (LLO), rendered cells poorly susceptible to DENV. We also established that in cells deficient for DPMS activity, viral RNA amplification is hampered and truncated oligosaccharides are transferred to the viral prM and E glycoproteins, affecting their proper folding. Overall, our study provides new insights into the host-dependent mechanisms of DENV infection and supports current therapeutic approaches using glycosylation inhibitors to treat DENV infection.IMPORTANCE Dengue disease, which is caused by dengue virus (DENV), has emerged as the most important mosquito-borne viral disease in humans and is a major global health concern. DENV encodes only few proteins and relies on the host cell machinery to accomplish its life cycle. The identification of the host factors important for DENV infection is needed to propose new targets for antiviral intervention. Using a genome-wide CRISPR-Cas9 screen, we identified DPM1 and -3, two subunits of the DPMS complex, as important host factors for the replication of DENV as well as other related viruses such as Zika virus. We established that DPMS complex plays dual roles during viral infection, both regulating viral RNA replication and promoting viral structural glycoprotein folding/stability. These results provide insights into the host molecules exploited by DENV and other flaviviruses to facilitate their life cycle.
Keywords: N-glycosylation; Zika virus; dengue fever; dengue virus; dolichol-phosphate mannose; glycoprotein folding.
Copyright © 2020 American Society for Microbiology.
Figures

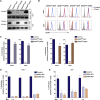
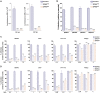





Similar articles
-
Genome-Wide CRISPR Screen Identifies RACK1 as a Critical Host Factor for Flavivirus Replication.J Virol. 2021 Nov 23;95(24):e0059621. doi: 10.1128/JVI.00596-21. Epub 2021 Sep 29. J Virol. 2021. PMID: 34586867 Free PMC article.
-
Genetic dissection of Flaviviridae host factors through genome-scale CRISPR screens.Nature. 2016 Jul 7;535(7610):159-63. doi: 10.1038/nature18631. Epub 2016 Jun 17. Nature. 2016. PMID: 27383987 Free PMC article.
-
RACK1 Associates with RNA-Binding Proteins Vigilin and SERBP1 to Facilitate Dengue Virus Replication.J Virol. 2022 Apr 13;96(7):e0196221. doi: 10.1128/jvi.01962-21. Epub 2022 Mar 10. J Virol. 2022. PMID: 35266803 Free PMC article.
-
Dolichol phosphate mannose synthase: a Glycosyltransferase with Unity in molecular diversities.Glycoconj J. 2017 Aug;34(4):467-479. doi: 10.1007/s10719-017-9777-4. Epub 2017 Jun 14. Glycoconj J. 2017. PMID: 28616799 Free PMC article. Review.
-
Dolichol-phosphate mannose synthase: structure, function and regulation.Biochim Biophys Acta. 2008 Jun;1780(6):861-8. doi: 10.1016/j.bbagen.2008.03.005. Epub 2008 Mar 14. Biochim Biophys Acta. 2008. PMID: 18387370 Review.
Cited by
-
Flavivirus-Host Interaction Landscape Visualized through Genome-Wide CRISPR Screens.Viruses. 2022 Sep 30;14(10):2164. doi: 10.3390/v14102164. Viruses. 2022. PMID: 36298718 Free PMC article. Review.
-
A Genome-Wide RNAi Screen Reveals Common Host-Virus Gene Signatures: Implication for Dengue Antiviral Drug Discovery.GEN Biotechnol. 2023 Apr 1;2(2):133-148. doi: 10.1089/genbio.2023.0001. Epub 2023 Apr 18. GEN Biotechnol. 2023. PMID: 37928776 Free PMC article.
-
Genetic glycoengineering in mammalian cells.J Biol Chem. 2021 Jan-Jun;296:100448. doi: 10.1016/j.jbc.2021.100448. Epub 2021 Feb 20. J Biol Chem. 2021. PMID: 33617880 Free PMC article. Review.
-
Beyond the Surface: Endocytosis of Mosquito-Borne Flaviviruses.Viruses. 2020 Dec 23;13(1):13. doi: 10.3390/v13010013. Viruses. 2020. PMID: 33374822 Free PMC article. Review.
-
Functional Roles and Host Interactions of Orthoflavivirus Non-Structural Proteins During Replication.Pathogens. 2025 Feb 12;14(2):184. doi: 10.3390/pathogens14020184. Pathogens. 2025. PMID: 40005559 Free PMC article. Review.
References
-
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi:10.1038/nature12060. - DOI - PMC - PubMed
-
- Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Muhammad Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, Tran HN, Bouckenooghe A, Chansinghakul D, Cortes M, Fanouillere K, Forrat R, Frago C, Gailhardou S, Jackson N, Noriega F, Plennevaux E, Wartel TA, Zambrano B, Saville M, CYD-TDV Dengue Vaccine Working Group. 2015. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 373:1195–1206. doi:10.1056/NEJMoa1506223. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

