The Clinical and Laboratory Standards Institute Subcommittee on Antimicrobial Susceptibility Testing: Background, Organization, Functions, and Processes
- PMID: 31915289
- PMCID: PMC7041576
- DOI: 10.1128/JCM.01864-19
The Clinical and Laboratory Standards Institute Subcommittee on Antimicrobial Susceptibility Testing: Background, Organization, Functions, and Processes
Abstract
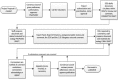
The Clinical and Laboratory Standards Institute (CLSI) Subcommittee on Antimicrobial Susceptibility Testing (AST SC) is a volunteer-led, multidisciplinary consensus body that develops and publishes standards and guidelines (among other products) for antimicrobial susceptibility testing (AST) methods and results interpretation in the United States and internationally. The Subcommittee (SC) meets face-to-face twice yearly, and its working groups (WGs) are active throughout the year via teleconferences. All meetings are open to the public. Participants include clinical microbiologists, infectious disease (ID) pharmacists, and infectious disease physicians representing the health care professions, government, and industry. Individuals who work for a company with a primary financial dependency on drug sales cannot serve as voting members, and well-defined conflict of interest polices are in place. In addition to developing and updating susceptibility breakpoints, the SC develops and validates new testing methods, provides guidance on how results should be interpreted and applied, sets quality control ranges, and educates users through seminars, symposia, and webinars. Based on its work, the SC publishes print and electronic standards and guidelines, including an annual update, the Performance Standards for Antimicrobial Susceptibility Testing (M100). This commentary will describe the background, organization, functions, and operational processes of the AST SC.
Copyright © 2020 American Society for Microbiology.
Figures
References
-
- Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial disk susceptibility tests, 13th ed CLSI standard M02 Clinical and Laboratory Standards Institute, Wayne, PA.
-
- Clinical and Laboratory Standards Institute. 2018. Performance standards for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 11th ed CLSI standard M07 Clinical and Laboratory Standards Institute, Wayne, PA.
-
- Clinical and Laboratory Standards Institute. 2018. Methods for antimicrobial susceptibility testing of anaerobic bacteria, 9th ed CLSI standard M11 Clinical and Laboratory Standards Institute, Wayne, PA.
-
- Clinical and Laboratory Standards Institute. 2016. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria, 3rd ed CLSI guideline M45 Clinical and Laboratory Standards Institute, Wayne, PA. - PubMed
-
- Clinical and Laboratory Standards Institute. 2020. Performance standards for antimicrobial susceptibility testing, 30th ed CLSI supplement M100 Clinical and Laboratory Standards Institute, Wayne, PA.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources