Randomised, double-blind, multicentre, mixed-methods, dose-escalation feasibility trial of mirtazapine for better treatment of severe breathlessness in advanced lung disease (BETTER-B feasibility)
- PMID: 31915308
- PMCID: PMC7029228
- DOI: 10.1136/thoraxjnl-2019-213879
Randomised, double-blind, multicentre, mixed-methods, dose-escalation feasibility trial of mirtazapine for better treatment of severe breathlessness in advanced lung disease (BETTER-B feasibility)
Abstract
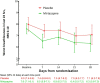
New treatments are required for severe breathlessness in advanced disease. We conducted a randomised feasibility trial of mirtazapine over 28 days in adults with a modified medical research council breathlessness scale score ≥3. Sixty-four patients were randomised (409 screened), achieving our primary feasibility endpoint of recruitment. Most patients had COPD or interstitial lung disease; 52 (81%) completed the trial. There were no differences between placebo and mirtazapine in tolerability or safety, and blinding was maintained. Worst breathlessness ratings at day 28 (primary clinical activity endpoint) were, 7.1 (SD 2.3, placebo) and 6.3 (SD 1.8, mirtazapine). A phase III trial of mirtazapine is indicated. Trial registration: ISRCTN 32236160; European Clinical Trials Database (EudraCT no: 2015-004064-11).
Keywords: COPD exacerbations; COPD pharmacology; drug reactions; emphysema; idiopathic pulmonary fibrosis; lung cancer; palliative care; psychology.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
