RodZ: a key-player in cell elongation and cell division in Escherichia coli
- PMID: 31915748
- PMCID: PMC6946637
- DOI: 10.3934/microbiol.2019.4.358
RodZ: a key-player in cell elongation and cell division in Escherichia coli
Abstract
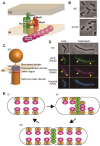
RodZ is required for determination of cell shape in rod-shaped bacterium, such as Escherichia coli. RodZ is a transmembrane protein and forms a supramolecular complex called the Rod complex with other proteins, such as MreB-actin and peptidoglycan synthesis enzymes (for e.g., PBP2). Deletion of the rodZ gene changes the cell shape from rod to round or ovoid. Another supramolecular complex called divisome that controls cell division mainly consists of FtsZ-tubulin. MreB directly interacts with FtsZ and this interaction is critical to trigger a transition from cell elongation to cell division. Recently, we found that RodZ also directly interacts with FtsZ, and RodZ recruits MreB to the divisome. Formation of the division ring, called Z ring, is delayed if RodZ does not interact with FtsZ, indicating that RodZ might facilitate the formation of the Z ring during the cell division process. In this mini-review, we have summarized the roles of RodZ in cell elongation and cell division, especially based on our recent study.
Keywords: FtsZ; MreB; Rod complex; RodZ; divisome; peptidoglycan.
© 2019 the Author(s), licensee AIMS Press.
Conflict of interest statement
Conflict of interest: The authors declare no conflict of interest in this review.
Figures
References
-
- Szwedziak P, Löwe J. Do the divisome and elongasome share a common evolutionary past? Curr Opin Microbiol. 2013;16:745–751. - PubMed
-
- Blaauwen den T, de Pedro MA, Nguyen-Distèche M, et al. Morphogenesis of rod-shaped sacculi. FEMS Microbiol Rev. 2008;32:321–344. - PubMed
-
- Figge RM, Divakaruni AV, Gober JW. MreB, the cell shape-determining bacterial actin homologue, co-ordinates cell wall morphogenesis in Caulobacter crescentus. Mol Microbiol. 2004;51:1321–1332. - PubMed
-
- Vats P, Shih YL, Rothfield L. Assembly of the MreB-associated cytoskeletal ring of Escherichia coli. Mol Microbiol. 2009;72:170–182. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
