A Phase 2b, Randomized, Double-blind, Placebo-Controlled Multicenter Study Evaluating Antiviral Effects, Pharmacokinetics, Safety, and Tolerability of Presatovir in Hematopoietic Cell Transplant Recipients with Respiratory Syncytial Virus Infection of the Lower Respiratory Tract
- PMID: 31915807
- PMCID: PMC7108198
- DOI: 10.1093/cid/ciz1167
A Phase 2b, Randomized, Double-blind, Placebo-Controlled Multicenter Study Evaluating Antiviral Effects, Pharmacokinetics, Safety, and Tolerability of Presatovir in Hematopoietic Cell Transplant Recipients with Respiratory Syncytial Virus Infection of the Lower Respiratory Tract
Abstract
Background: Presatovir significantly reduced nasal viral load, signs, and symptoms of respiratory syncytial virus (RSV) infection in a human challenge study. We evaluated presatovir in hematopoietic-cell transplant (HCT) recipients with RSV lower respiratory tract infection (LRTI).
Methods: Patients with confirmed RSV in upper and lower respiratory tract and new chest X-ray abnormalities were randomized (1:1), stratified by supplemental oxygen and ribavirin use, to receive oral presatovir 200 mg or placebo every 4 days for 5 doses. The primary endpoint was time-weighted average change in nasal RSV viral load through day 9. Secondary endpoints included supplemental oxygen-free days, incident respiratory failure requiring mechanical ventilation, and all-cause mortality.
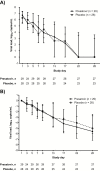
Results: From January 31, 2015, to March 20, 2017, 60 patients from 17 centers were randomized (31 presatovir, 29 placebo); 59 received study treatment (50 allogeneic, 9 autologous HCT). In the efficacy population (29 presatovir, 28 placebo), presatovir treatment did not significantly reduce time-weighted average change in viral load (-1.12 vs -1.09 log10 copies/mL; treatment difference -0.02 log10 copies/mL, 95% confidence interval: -.62, .57; P = .94), median supplemental oxygen-free days (26 vs 28 days, P = .84), incident respiratory failure (10.3 vs 10.7%, P = .98), or all-cause mortality (0 vs 7.1%, P = .19) versus placebo. Adverse events were similar between arms (presatovir 80%, placebo 79%). Resistance-associated substitutions in RSV fusion protein emerged in 6/29 presatovir-treated patients.
Conclusions: Presatovir treatment was well tolerated in HCT patients with RSV LRTI but did not improve virologic or clinical outcomes versus placebo.
Clinical trials registration: www.clinicaltrials.gov, NCT02254421; EudraCT, #2014-002475-29.
Keywords: Presatovir; hematopoietic cell transplant; lower respiratory tract infection; respiratory syncytial virus.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

Comment in
-
Clinical Development of Respiratory Syncytial Virus Antivirals-What We Can Learn From Oseltamivir.Clin Infect Dis. 2020 Dec 31;71(11):2796-2798. doi: 10.1093/cid/ciz1169. Clin Infect Dis. 2020. PMID: 31915814 No abstract available.
References
-
- Whimbey E, Champlin RE, Couch RB, et al. . Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin Infect Dis 1996; 22:778–82. - PubMed

