Androgens Modulate Rat Granulosa Cell Steroidogenesis
- PMID: 31916094
- PMCID: PMC7539820
- DOI: 10.1007/s43032-019-00099-0
Androgens Modulate Rat Granulosa Cell Steroidogenesis
Abstract
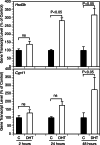
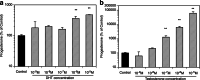
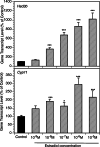
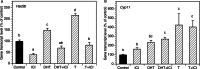
Paracrine interactions between ovarian theca-interstitial cells (TICs) and granulosa cells (GCs) play an important role in the regulation of follicular steroidogenesis. Androgens serve as substrates for aromatization as well as affect GC function. This study evaluated the effects of co-culture of GC with TICs and the role of testosterone (T) and 5-alpha-dihydrotestosterone (DHT), and estradiol (E2) in modulation of GC expression of genes involved in the production of progesterone: 3β-hydroxysteroid dehydrogenase/Δ5-4 isomerase (Hsd3b) and cholesterol side-chain cleavage (Cyp11). GCs obtained from immature Sprague-Dawley rats and were cultured in chemically defined media without or with TICs, DHT, or T. Hsd3b and Cyp11 transcripts were analyzed by qt-PCR. Co-culture of GCs with TICs stimulated Hsd3b and CYP11 expression in GCs. DHT and T induced a concentration-dependent upregulation of Hsd3b and CYP11 expression, as well as increased progesterone concentrations in spent media. E2 also increased expression of Hsd3b, and Cyp11. Effects of androgens were abrogated in the presence of an anti-androgen bicalutamide and the antiestrogen ICI 182780 (ICI). In conclusion, present findings demonstrate that androgens upregulate production of progesterone in GCs; these effects are likely due to a combination of direct action on androgen receptors and effects mediated by estrogen receptors.
Keywords: Androgens; Gene expression; Granulosa cells; Rat.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

