Declining BRCA-Mediated DNA Repair in Sperm Aging and its Prevention by Sphingosine-1-Phosphate
- PMID: 31916095
- PMCID: PMC7065969
- DOI: 10.1007/s43032-019-00098-1
Declining BRCA-Mediated DNA Repair in Sperm Aging and its Prevention by Sphingosine-1-Phosphate
Abstract
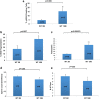
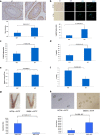
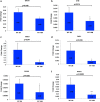
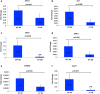
Recent data suggest that paternal age can have major impact on reproductive outcomes, and with increased age, there is increased likelihood of chromosomal abnormalities in the sperm. Here, we studied DNA damage and repair as a function of male aging and assessed whether sphingosine-1-phosphate (S1P), a ceramide-induced death inhibitor, can prevent sperm aging by enhancing DNA double-strand breaks (DSB) repair. We observed a significant increase in DNA damage with age and this increase was associated with a decline in the expression of key DNA DSB repair genes in mouse sperm. The haploinsufficiency of BRCA1 male mice sperm showed significantly increased DNA damage and apoptosis, along with decreased chromatin integrity when compared to similar age wild type (WT) mice. Furthermore, haploinsufficiency of BRCA1 male mice had lower sperm count and smaller litter size when crossed with WT females. The resulting embryos had a higher probability of growth arrest and reduced implantation. S1P treatment decreased genotoxic-stress-induced DNA damage in sperm and enhanced the expressions of key DNA repair genes such as BRCA1. Co-treatment with an ATM inhibitor reversed the effects of S1P, implying that the impact of S1P on DNA repair is via the ATM-mediated pathway. Our findings indicate a key role for DNA damage repair mechanism in the maintenance of sperm integrity and suggest that S1P can improve DNA repair in sperm. Further translational studies are warranted to determine the clinical significance of these findings and whether S1P can delay male reproductive aging. There is mounting evidence that sperm quality declines with age, similar to that of the oocyte. However, the reasons behind this decline are poorly understood and there is no medical intervention to improve sperm quality. Our study suggests a strong role for DNA damage repair in maintenance of sperm quality, and for the first time, a potential pharmaceutical approach to prevent sperm aging.
Keywords: Aging; DNA fragmentation; Gene expression; Sperm.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
BRCA-related ATM-mediated DNA double-strand break repair and ovarian aging.Hum Reprod Update. 2020 Jan 1;26(1):43-57. doi: 10.1093/humupd/dmz043. Hum Reprod Update. 2020. PMID: 31822904 Free PMC article. Review.
-
Paternal influence of sperm DNA integrity on early embryonic development.Hum Reprod. 2014 Nov;29(11):2402-12. doi: 10.1093/humrep/deu228. Epub 2014 Sep 8. Hum Reprod. 2014. PMID: 25205757
-
Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans.Sci Transl Med. 2013 Feb 13;5(172):172ra21. doi: 10.1126/scitranslmed.3004925. Sci Transl Med. 2013. PMID: 23408054 Free PMC article.
-
Sphingosine 1-phosphate promotes mesenchymal stem cell-mediated cardioprotection against myocardial infarction via ERK1/2-MMP-9 and Akt signaling axis.Life Sci. 2018 Dec 15;215:31-42. doi: 10.1016/j.lfs.2018.10.047. Epub 2018 Oct 24. Life Sci. 2018. PMID: 30367841
-
Therapeutic exploitation of tumor cell defects in homologous recombination.Anticancer Agents Med Chem. 2008 May;8(4):448-60. doi: 10.2174/187152008784220267. Anticancer Agents Med Chem. 2008. PMID: 18473729 Review.
Cited by
-
DNA damage control then and now: a matter of life or death.J Assist Reprod Genet. 2020 Jul;37(7):1509-1510. doi: 10.1007/s10815-020-01889-1. J Assist Reprod Genet. 2020. PMID: 32671733 Free PMC article. No abstract available.
-
Sperm Lipid Markers of Male Fertility in Mammals.Int J Mol Sci. 2021 Aug 16;22(16):8767. doi: 10.3390/ijms22168767. Int J Mol Sci. 2021. PMID: 34445473 Free PMC article. Review.
-
The role of declining ataxia-telangiectasia-mutated (ATM) function in oocyte aging.Cell Death Discov. 2024 Jun 25;10(1):302. doi: 10.1038/s41420-024-02041-z. Cell Death Discov. 2024. PMID: 38914566 Free PMC article.
-
Male aging in germ cells: What are we inheriting?Genet Mol Biol. 2025 Feb 18;47Suppl 1(Suppl 1):e20240052. doi: 10.1590/1678-4685-GMB-2024-0052. eCollection 2025. Genet Mol Biol. 2025. PMID: 39969160 Free PMC article.
-
Epimedium protects against dyszoospermia in mice with Pex3 knockout by exerting antioxidant effects and regulating the expression level of P16.Cell Death Dis. 2022 Jan 20;13(1):69. doi: 10.1038/s41419-021-04435-8. Cell Death Dis. 2022. PMID: 35058429 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

