Mapping neural dynamics underlying saccade preparation and execution and their relation to reaction time and direction errors
- PMID: 31916374
- PMCID: PMC7268073
- DOI: 10.1002/hbm.24922
Mapping neural dynamics underlying saccade preparation and execution and their relation to reaction time and direction errors
Abstract
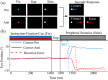
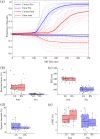
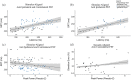
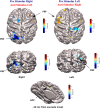
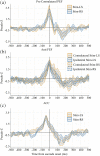
Our ability to control and inhibit automatic behaviors is crucial for negotiating complex environments, all of which require rapid communication between sensory, motor, and cognitive networks. Here, we measured neuromagnetic brain activity to investigate the neural timing of cortical areas needed for inhibitory control, while 14 healthy young adults performed an interleaved prosaccade (look at a peripheral visual stimulus) and antisaccade (look away from stimulus) task. Analysis of how neural activity relates to saccade reaction time (SRT) and occurrence of direction errors (look at stimulus on antisaccade trials) provides insight into inhibitory control. Neuromagnetic source activity was used to extract stimulus-aligned and saccade-aligned activity to examine temporal differences between prosaccade and antisaccade trials in brain regions associated with saccade control. For stimulus-aligned antisaccade trials, a longer SRT was associated with delayed onset of neural activity within the ipsilateral parietal eye field (PEF) and bilateral frontal eye field (FEF). Saccade-aligned activity demonstrated peak activation 10ms before saccade-onset within the contralateral PEF for prosaccade trials and within the bilateral FEF for antisaccade trials. In addition, failure to inhibit prosaccades on anti-saccade trials was associated with increased activity prior to saccade onset within the FEF contralateral to the peripheral stimulus. This work on dynamic activity adds to our knowledge that direction errors were due, at least in part, to a failure to inhibit automatic prosaccades. These findings provide novel evidence in humans regarding the temporal dynamics within oculomotor areas needed for saccade programming and the role frontal brain regions have on top-down inhibitory control.
Keywords: antisaccade; frontal cortex; inhibition; magnetoencephalography; parietal cortex.
© 2020 The Authors. Human Brain Mapping published by Wiley Periodicals, Inc.
Figures


References
-
- Amador, N. , Schlag‐Rey, M. , & Schlag, J. (2004). Primate Antisaccade. II. Supplementary eye field neuronal activity predicts correct performance. Journal of Neurophysiology, 91, 1672–1689. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

