Effect of Food on the Pharmacokinetics of Quizartinib
- PMID: 31916418
- PMCID: PMC7027461
- DOI: 10.1002/cpdd.770
Effect of Food on the Pharmacokinetics of Quizartinib
Abstract
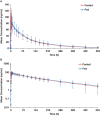
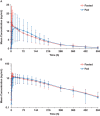
Quizartinib is an oral, highly potent, and selective type II FMS-like tyrosine kinase 3 inhibitor in development for acute myeloid leukemia. This parallel-group study evaluated potential food effects on quizartinib absorption in healthy subjects who received a single 30-mg dose after overnight fasting (n = 34) or a high-fat, high-calorie meal (n = 30). Blood samples were collected through 504 hours after dosing, and pharmacokinetic parameters calculated were maximum observed concentration (Cmax ) and area under plasma concentration-time curve from time 0 to last quantifiable concentration (AUClast ) and from time 0 to infinity (AUCinf ). Mean quizartinib pharmacokinetic profiles were similar under fasted and fed conditions. The geometric least squares means ratios (%) for fed/fasted and associated 90% confidence intervals (CIs) for Cmax , AUClast , and AUCinf were 91.58 (82.15-102.08), 105.39 (90.79-122.35), and 108.39 (91.54-128.34), respectively. The 90%CI for the ratio fell within the 80% to 125% limits for Cmax and AUClast , with 90%CI for AUCinf slightly outside the limits (ie, 128%). Food delayed quizartinib time to Cmax by 2 hours. All adverse events were either mild or moderate; no discontinuations due to adverse events occurred. Based on these results, quizartinib can be administered without regard to food.
Keywords: AML; FLT3; TKI; food effect; quizartinib.
© 2020 The Authors. Clinical Pharmacology in Drug Development published by Wiley Periodicals, Inc. on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
J.L. and M.H. were employees of Daiichi Sankyo, Inc. and Ambit Biosciences at the time this study was conducted. J.M. is an employee of Daiichi Sankyo, Inc. D.T. was an employee of Daiichi Sankyo, Inc. and Ambit Biosciences at the time this study was conducted. G.G. was an employee of Daiichi Sankyo, Inc. at the time this study was conducted and has received stock or other ownership in and travel/accommodations/expenses from Daiichi Sankyo. M.K. has no conflicts to disclose.
Figures

References
-
- Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136‐1152. - PubMed
-
- Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98(6):1752‐1759. - PubMed
-
- Chao Q, Sprankle KG, Grotzfeld RM, et al. Identification of N‐(5‐tert‐butyl‐isoxazol‐3‐yl)‐N′‐{4‐[7‐(2‐morpholin‐4‐yl‐ethoxy)imidazo[2,1‐b][1,3]benzothiazol‐2‐yl]phenyl}urea dihydrochloride (AC220), a uniquely potent, selective, and efficacious FMS‐like tyrosine kinase‐3 (FLT3) inhibitor. J Med Chem. 2009;52(23):7808‐7816. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

